| Cross-matching ID |
- PubChem CID
- 54671203
- PubChem SID
-
9187
; 596105
; 7979130
; 11335520
; 11360759
; 11363701
; 11366263
; 11368825
; 11371256
; 11374272
; 11376987
; 11461731
; 11483982
; 11487959
; 11490224
; 11492342
; 11494621
; 14759559
; 14881664
; 22391429
; 26755247
; 29279579
; 39289952
; 39460532
; 46171207
; 46487965
; 46506491
; 47216696
; 47440169
; 47810665
; 47885321
; 47885322
; 47959651
; 49994878
; 50071302
; 50110882
; 53787282
; 56310727
; 56310911
; 56310919
; 56311074
; 56311250
; 56311566
; 56311567
; 56311897
; 56311928
; 56312054
; 56312284
; 56312312
; 56312315
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0S0LZ
- Formula
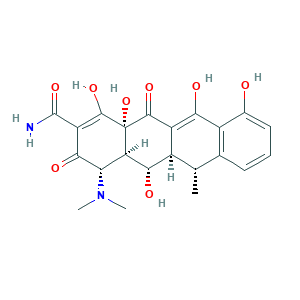
- C22H24N2O8
- Canonical SMILES
- CC1C2C(C3C(C(=O)C(=C(C3(C(=O)C2=C(C4=C1C=CC=C4O)O)O)O)C(=O)N)N(C)C)O
- InChI
- 1S/C22H24N2O8/c1-7-8-5-4-6-9(25)11(8)16(26)12-10(7)17(27)14-15(24(2)3)18(28)13(21(23)31)20(30)22(14,32)19(12)29/h4-7,10,14-15,17,25-27,30,32H,1-3H3,(H2,23,31)/t7-,10+,14+,15-,17-,22-/m0/s1
- InChIKey
- SGKRLCUYIXIAHR-AKNGSSGZSA-N
|