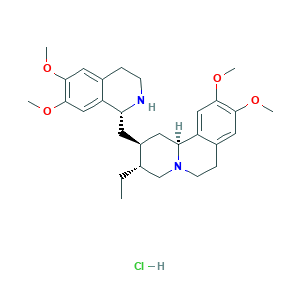
| Synonyms |
Cephaeline methyl ether HCl; Emetine; Emetine hydrochloride; Emetine monohydrochloride; Opera_ID_1460; Q-100155; SCHEMBL636599; ST51014995; 10-[((1R)-6,7-dimethoxy(1,2,3,4-tetrahydroisoquinolyl))methyl](10S,11aS,9R)-9- ethyl-2,3-dimethoxy-5,6,7,11a-tetrahydropiperidino[2,1-a]isoquinoline, chlorid e; EMETINE; 14198-59-5; AC1O7FP4; AKOS024374935; CHEMBL513000; Cephaeline methyl ether hydrochloride; DTXSID80424947; Emetan,7',10,11-tetramethoxy-, dihydrochloride; MLS000028478; NSC-33669; NSC-752340; NSC33669; NSC752340; SMR000058444
|
| Cross-matching ID |
- PubChem CID
- 6603320
- CAS Number
-
- TTD Drug ID
- D07DJQ
- Formula
- C29H41ClN2O4
- Canonical SMILES
- CCC1CN2CCC3=CC(=C(C=C3C2CC1CC4C5=CC(=C(C=C5CCN4)OC)OC)OC)OC.Cl
- InChI
- 1S/C29H40N2O4.ClH/c1-6-18-17-31-10-8-20-14-27(33-3)29(35-5)16-23(20)25(31)12-21(18)11-24-22-15-28(34-4)26(32-2)13-19(22)7-9-30-24;/h13-16,18,21,24-25,30H,6-12,17H2,1-5H3;1H/t18-,21-,24+,25-;/m0./s1
- InChIKey
- HUEYSSLYFJVUIS-MRFSYGAJSA-N
|