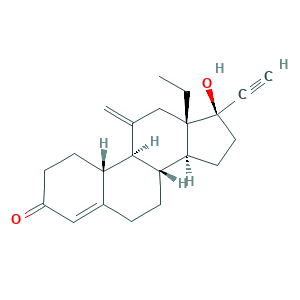
| Synonyms |
Etonogestrel (USAN/INN); Implanon; Implanon (TN); ORG 3236; Org-3236; etonogestrelum; nexplanon; (8S,9S,10R,13S,14S,17R)-13-ethyl-17-ethynyl-17-hydroxy-11-methylidene-2,6,7,8,9,10,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-3-one; 13-Ethyl-17-hydroxy-11-methylene-18,19-dinor-17alpha-pregn-4-en-20-yn-3-one; 17-Ethinyl-17-beta-hydroxy-18-methyl-11-methylene-4-estren-3-one; 3-Keto-desogestrel; 3-Ketodesogestrel; 3-Oxodesogestrel; 304GTH6RNH; 54048-10-1; CHEBI:50777; ETONOGESTREL; EINECS 258-936-2; UNII-304GTH6RNH
|
| Cross-matching ID |
- PubChem CID
- 6917715
- PubChem SID
-
12012881
; 14752897
; 14777213
; 17194516
; 43529146
; 46505321
; 47206056
; 53789691
; 56352980
; 57371661
; 74860218
; 103565890
; 114786833
; 126686585
; 135004061
; 136314851
; 137005554
; 140504091
; 144206191
; 162262948
; 164152391
; 164826700
; 175268832
; 179150591
; 193126307
; 198993353
; 210279789
; 210282112
; 223365925
; 223673211
; 226489730
; 241094452
; 249582869
; 252349777
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D02KIU
- Formula
- C22H28O2
- Canonical SMILES
- CCC12CC(=C)C3C(C1CCC2(C#C)O)CCC4=CC(=O)CCC34
- InChI
- 1S/C22H28O2/c1-4-21-13-14(3)20-17-9-7-16(23)12-15(17)6-8-18(20)19(21)10-11-22(21,24)5-2/h2,12,17-20,24H,3-4,6-11,13H2,1H3/t17-,18-,19-,20+,21-,22-/m0/s1
- InChIKey
- GCKFUYQCUCGESZ-BPIQYHPVSA-N
|