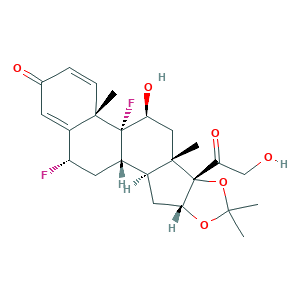
| Cross-matching ID |
- PubChem CID
- 6215
- PubChem SID
-
221901
; 398387
; 855535
; 7848887
; 8153903
; 11446394
; 12173607
; 14906599
; 14931123
; 24894978
; 25653430
; 29225213
; 46506244
; 50019426
; 56422130
; 57288777
; 57323264
; 57654197
; 78519291
; 87560013
; 92309170
; 92717058
; 103261079
; 103804966
; 104170060
; 104253637
; 104311430
; 117585830
; 121362472
; 124799361
; 126592956
; 126622532
; 126654102
; 131465784
; 134222020
; 134338094
; 134970880
; 136166211
; 137004589
; 139809249
; 144204420
; 144212681
; 152102563
; 160843984
; 160963936
; 170464630
; 172080685
; 174530283
; 175266910
; 175612171
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D02QJH
- Formula
- C24H30F2O6
- Canonical SMILES
- CC1(OC2CC3C4CC(C5=CC(=O)C=CC5(C4(C(CC3(C2(O1)C(=O)CO)C)O)F)C)F)C
- InChI
- 1S/C24H30F2O6/c1-20(2)31-19-9-13-14-8-16(25)15-7-12(28)5-6-21(15,3)23(14,26)17(29)10-22(13,4)24(19,32-20)18(30)11-27/h5-7,13-14,16-17,19,27,29H,8-11H2,1-4H3/t13-,14-,16-,17-,19+,21-,22-,23-,24+/m0/s1
- InChIKey
- FEBLZLNTKCEFIT-VSXGLTOVSA-N
|