| Cross-matching ID |
- PubChem CID
- 15209
- PubChem SID
-
855862
; 7847394
; 7979291
; 8161408
; 10321974
; 11466673
; 11467793
; 11486306
; 29283181
; 46505159
; 47365453
; 47440538
; 47960000
; 48185237
; 49698604
; 50019672
; 50104126
; 56424122
; 57328916
; 71831477
; 80662145
; 85787964
; 92125615
; 103770995
; 103914443
; 104336512
; 121363286
; 124800386
; 126651364
; 126691320
; 134221913
; 134337714
; 134979670
; 135925245
; 137008617
; 141792094
; 144204060
; 144212928
; 160964187
; 162184963
; 162198487
; 170465298
; 175268451
; 175611950
; 179116699
; 184580842
; 196108775
; 223365941
; 223660113
; 226396638
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0Y2YP
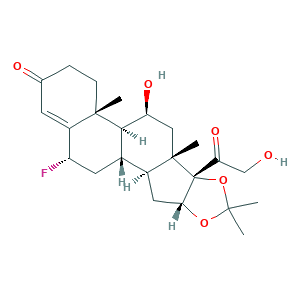
- Formula
- C24H33FO6
- Canonical SMILES
- CC1(OC2CC3C4CC(C5=CC(=O)CCC5(C4C(CC3(C2(O1)C(=O)CO)C)O)C)F)C
- InChI
- 1S/C24H33FO6/c1-21(2)30-19-9-14-13-8-16(25)15-7-12(27)5-6-22(15,3)20(13)17(28)10-23(14,4)24(19,31-21)18(29)11-26/h7,13-14,16-17,19-20,26,28H,5-6,8-11H2,1-4H3/t13-,14-,16-,17-,19+,20+,22-,23-,24+/m0/s1
- InChIKey
- POPFMWWJOGLOIF-XWCQMRHXSA-N
|