| General Information of Drug (ID:
DR0869) |
| Drug Name |
Indenolol
|
| Synonyms |
Indenolol; Indenolol [INN]; Indenolol [BAN:INN]; Indenolol [INN:BAN]; Indenololum; Indenololum [INN-Latin]; Sch 28316 Z; Sch 28316Z; Securpres; 1-(4-Indenyloxy)-3-isopropylamino-2-propanol; 1-(Inden-4(or 7)-yloxy)-3-(isopropylamino)-2-propanol; 106656-86-4; 2-Propanol, 1-(1H-inden-4-yloxy)-3-((1-methylethyl)amino)-; 2-Propanol, 1-(1H-inden-4-yloxy)-3-[(1-methylethyl)amino]-; 4-(2-Hydroxy-3-isopropylaminopropoxy)indene; 60607-68-3; AC1L2H51; AC1Q77JA; ACMC-20macf; C15H27NO2; CHEMBL153585; EINECS 262-323-5; SCHEMBL79021; YB-2
|
| Indication |
Cardiac arrhythmia
[ICD11: BC65]
|
Phase 4
|
[1]
|
| Structure |
|
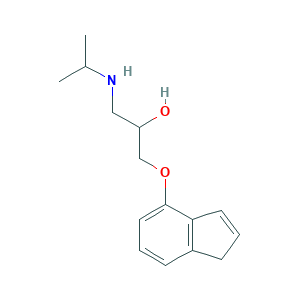 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
247.33 |
Topological Polar Surface Area |
41.5 |
| Heavy Atom Count |
18 |
Rotatable Bond Count |
6 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
3 |
| Cross-matching ID |
- PubChem CID
- 71955
- CAS Number
-
- Formula
- C15H21NO2
- Canonical SMILES
- CC(C)NCC(COC1=CC=CC2=C1C=CC2)O
- InChI
- 1S/C15H21NO2/c1-11(2)16-9-13(17)10-18-15-8-4-6-12-5-3-7-14(12)15/h3-4,6-8,11,13,16-17H,5,9-10H2,1-2H3
- InChIKey
- MPGBPFMOOXKQRX-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


