| General Information of Drug (ID:
DR0876) |
| Drug Name |
Indoramin
|
| Synonyms |
Indoramina; Indoramina [INN-Spanish]; Indoramine; Indoramine [INN-French]; Indoraminum [INN-Latin]; JXZZEXZZKAWDSP-UHFFFAOYSA-N; Baratol; INDORAMIN; WY 21901; Wy-21901; 0Z802HMY7H; 26844-12-2; 3-(2-(4-Benzamidopiperid-1-yl)ethyl)indole; BRN 0494035; Benzamide, N-(1-(2-(1H-indol-3-yl)ethyl)-4-piperidinyl)-; Benzamide, N-(1-(2-indol-3-ylethyl)-4-piperidyl)-; C22H25N3O; CHEMBL279516; EINECS 248-041-5; N-(1-(2-(1H-Indol-3-yl)ethyl)-4-piperidinyl)benzamide; N-(1-(2-Indol-3-ylethyl)-4-piperidyl)benzamide; UNII-0Z802HMY7H
|
| Indication |
Essential hypertension
[ICD11: BA00]
|
Phase 4
|
[1]
|
| Structure |
|
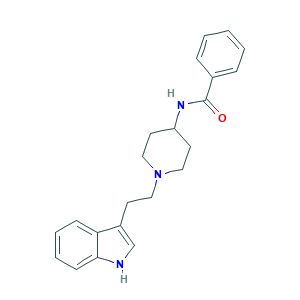 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
347.5 |
Topological Polar Surface Area |
48.1 |
| Heavy Atom Count |
26 |
Rotatable Bond Count |
5 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
2 |
| Cross-matching ID |
- PubChem CID
- 33625
- PubChem SID
-
3145511
; 4954321
; 7979608
; 8173213
; 11393053
; 14778365
; 26534678
; 29217568
; 34675413
; 47206378
; 47227712
; 47301929
; 47451424
; 47747528
; 47971029
; 48345790
; 49671666
; 49876989
; 50603739
; 57311546
; 85209952
; 89765137
; 92270335
; 103006849
; 103194521
; 103941454
; 104316511
; 117564550
; 124638943
; 125163373
; 125433253
; 128343844
; 134224549
; 134340978
; 134997857
; 135650391
; 136050062
; 137248543
; 141645446
; 152133947
; 162185578
; 179150251
; 184547504
; 187071875
; 198950576
; 223519058
; 224810415
; 226433258
; 226433259
; 241046546
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D02LHR
- Formula
- C22H25N3O
- Canonical SMILES
- C1CN(CCC1NC(=O)C2=CC=CC=C2)CCC3=CNC4=CC=CC=C43
- InChI
- 1S/C22H25N3O/c26-22(17-6-2-1-3-7-17)24-19-11-14-25(15-12-19)13-10-18-16-23-21-9-5-4-8-20(18)21/h1-9,16,19,23H,10-15H2,(H,24,26)
- InChIKey
- JXZZEXZZKAWDSP-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


