| Synonyms |
Ixabepilone; Ixempra; Aza-epothilone B; Azaepothilone B; K27005NP0A; 17-oxa-4-azabicyclo(14.1.0)heptadecane-5,9-dione, 7,11-dihydroxy-8,8,10,12,16-pentamethyl-3-((1E)-1-methyl-2-(2-methyl-4-thiazolyl)ethenyl)-, (1S,3S,7S,10R,11S,12S,16R)-; 1S,3S,7S,10R,11S,12S,16R)-7,11-Dihydroxy-8,8,10,12,16-pentamethyl-3-((1E)-1-methyl-2-(2-methylthiazol-4-yl)ethenyl)-17-oxa-4-azabicyclo(14.1.0)heptadecane-5,9-dione; 219989-84-1; BMS 247550; BMS 247550-01; BMS 247550-1; BMS-247550; BMS-247550-01; CHEBI:63605; NSC747973; UNII-K27005NP0A
|
| Cross-matching ID |
- PubChem CID
- 6445540
- PubChem SID
-
11969810
; 14786763
; 14933557
; 34184371
; 47206488
; 49684213
; 49999545
; 57369862
; 76567192
; 91148449
; 103771488
; 114573104
; 126623101
; 126665598
; 127325936
; 127325937
; 127325938
; 127325939
; 134223036
; 135135146
; 135610851
; 137240334
; 137248706
; 140173305
; 152258135
; 160646974
; 162202446
; 175267394
; 176484667
; 178103430
; 179150167
; 184022005
; 184529017
; 184812276
; 221678841
; 223664545
; 226399596
; 228058699
; 241053227
; 248880927
; 251910828
; 252448545
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0W2EK
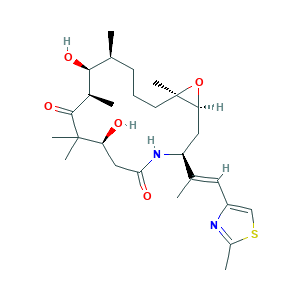
- Formula
- C27H42N2O5S
- Canonical SMILES
- CC1CCCC2(C(O2)CC(NC(=O)CC(C(C(=O)C(C1O)C)(C)C)O)C(=CC3=CSC(=N3)C)C)C
- InChI
- 1S/C27H42N2O5S/c1-15-9-8-10-27(7)22(34-27)12-20(16(2)11-19-14-35-18(4)28-19)29-23(31)13-21(30)26(5,6)25(33)17(3)24(15)32/h11,14-15,17,20-22,24,30,32H,8-10,12-13H2,1-7H3,(H,29,31)/b16-11+/t15-,17+,20-,21-,22-,24-,27+/m0/s1
- InChIKey
- FABUFPQFXZVHFB-PVYNADRNSA-N
|