Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR0932) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Levobetaxolol
|
|||||
| Synonyms |
Levobetaxolol; Levobetaxolol [INN]; levobetaxololum; (-)-Betaxolol; (2S)-1-[4-[2-(cyclopropylmethoxy)ethyl]phenoxy]-3-(propan-2-ylamino)propan-2-ol; (2S)-1-{4-[2-(cyclopropylmethoxy)ethyl]phenoxy}-3-(propan-2-ylamino)propan-2-ol; (S)-(-)-Betaxolol; (S)-1-(isopropylamino)-3-[p-(cyclopropylmethoxyethyl)phenoxy]-2-propanol; (S)-Betaxolol; 2-Propanol, 1-(4-(2-(cyclopropylmethoxy)ethyl)phenoxy)-3-((1-methylethyl)amino)-, (2S)-; 75O9XHA4TU; 93221-48-8; AC1L1TO2; AC1Q58SH; CHEBI:59254; SCHEMBL23532; UNII-75O9XHA4TU
|
|||||
| Indication | Glaucoma [ICD11: 9C61] | Approved | [1] | |||
| Structure |
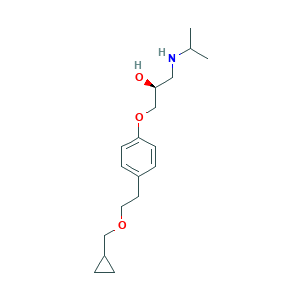 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 307.4 | Topological Polar Surface Area | 50.7 | ||
| Heavy Atom Count | 22 | Rotatable Bond Count | 11 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 4 | |||
| Cross-matching ID |
|
|||||
| The Predicted Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Predicted Drug Metabolites (PDM) of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

