| General Information of Drug (ID:
DR0992) |
| Drug Name |
Lumefantrine
|
| Synonyms |
Lumefantrine [INN]; Lumefantrine [USAN]; Benflumetol; ZUV4B00D9P; dl-Benflumelol; (+-)-2,7-Dichloro-9-((Z)-p-chlorobenzylidene)-alpha-((dibutylamino)methyl)fluorene-4-methanol; (Z)-2-(Dibutylamino)-1-(2,7-dichloro-9-(4-chlorobenzylidene)-9H-fluoren-4-yl)ethanol; 01NP22J3SV; 2-(dibutylamino)-1-[(9Z)-2,7-dichloro-9-[(4-chlorophenyl)methylidene]fluoren-4-yl]ethanol; 82186-77-4; C30H32Cl3NO; CHEBI:156095; DSSTox_CID_26663; HSDB 7210; MFCD05662268; NCGC00167490-01; UNII-01NP22J3SV; UNII-ZUV4B00D9P
|
| Indication |
Malaria
[ICD11: 1F40]
|
Approved
|
[1]
|
| Structure |
|
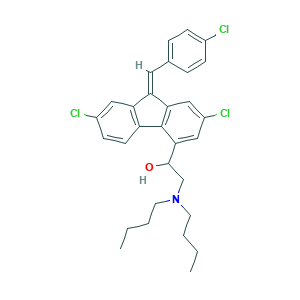 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
528.9 |
Topological Polar Surface Area |
23.5 |
| Heavy Atom Count |
35 |
Rotatable Bond Count |
10 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
2 |
| Cross-matching ID |
- PubChem CID
- 6437380
- PubChem SID
-
11967754
; 14885370
; 43030476
; 47205892
; 50065264
; 50112743
; 57367877
; 75869758
; 81092876
; 85352679
; 92720559
; 93620655
; 99007025
; 99443260
; 103215332
; 114553360
; 121360926
; 126619887
; 126647975
; 126666837
; 131327225
; 131332294
; 134222610
; 135065105
; 135684152
; 136375542
; 136949638
; 137249473
; 144206044
; 152057600
; 160967877
; 162170615
; 163656731
; 164825530
; 170465388
; 172090904
; 173896963
; 174477484
; 174530872
; 175612331
; 176235915
; 179148062
; 179322661
; 180372451
; 184017215
; 184548301
; 185974013
; 187072118
; 196111343
; 198991533
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D06MQM
- Formula
- C30H32Cl3NO
- Canonical SMILES
- CCCCN(CCCC)CC(C1=CC(=CC2=C1C3=C(C2=CC4=CC=C(C=C4)Cl)C=C(C=C3)Cl)Cl)O
- InChI
- 1S/C30H32Cl3NO/c1-3-5-13-34(14-6-4-2)19-29(35)28-18-23(33)17-27-25(15-20-7-9-21(31)10-8-20)26-16-22(32)11-12-24(26)30(27)28/h7-12,15-18,29,35H,3-6,13-14,19H2,1-2H3/b25-15-
- InChIKey
- DYLGFOYVTXJFJP-MYYYXRDXSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


