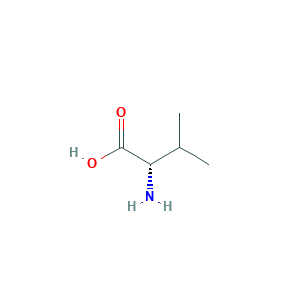
| Synonyms |
Butanoic acid, 2-amino-3-methyl-; H-Val-OH; L(+)-alpha-Aminoisovaleric acid; L-valine; VALINE, L-; Valina [Spanish]; Valine (VAN); Valine [USAN:INN]; Valinum [Latin]; valine; (2S)-2-amino-3-methylbutanoic acid; (S)-2-Amino-3-methylbutanoic acid; (S)-2-Amino-3-methylbutyric acid; (S)-Valine; (S)-alpha-Amino-beta-methylbutyric acid; 2-Amino-3-methylbutanoic acid; 2-Amino-3-methylbutyric acid; 2-Amino-3-methylbutyric acid, (S)-; 72-18-4; Butanoic acid, 2-amino-3-methyl-, (S)-; L-alpha-Amino-beta-methylbutyric acid
|
| Cross-matching ID |
- PubChem CID
- 6287
- PubChem SID
-
3483
; 595252
; 833261
; 841335
; 3134856
; 7847107
; 7891042
; 8025201
; 8143572
; 8153964
; 10533892
; 11528406
; 14710672
; 14716652
; 15119814
; 15218854
; 17424984
; 24890028
; 24900695
; 24900696
; 24900749
; 24902117
; 29204662
; 29225281
; 46393202
; 46504886
; 49748671
; 57323321
; 57653624
; 57654728
; 79527381
; 81044512
; 83100465
; 85164900
; 85267077
; 87350316
; 87577814
; 87692913
; 87692918
; 92297810
; 96100169
; 99224278
; 103059097
; 103059671
; 103224325
; 104234101
; 104311628
; 117365452
; 117689352
; 124384987
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0LL5V
- Formula
- C5H11NO2
- Canonical SMILES
- CC(C)C(C(=O)O)N
- InChI
- 1S/C5H11NO2/c1-3(2)4(6)5(7)8/h3-4H,6H2,1-2H3,(H,7,8)/t4-/m0/s1
- InChIKey
- KZSNJWFQEVHDMF-BYPYZUCNSA-N
|