| General Information of Drug (ID:
DR1033) |
| Drug Name |
Mesoridazine
|
| Synonyms |
Mesoridazina; Mesoridazina [INN-Spanish]; Mesoridazinum; Mesoridazinum [INN-Latin]; NC-123; Serentil; T-2-SO; THD-2-SO; TPS 23; TPS-23; Calodal; Lidanar; Lidanil; Thioridazien thiomethyl sulfoxide; Thioridazine monosulfoxide analog; Thioridazine thiomethyl sulfoxide; Thioridazine-2-sulfoxide; mesoridazine; 10-(2-(1-Methyl-2-piperidinyl)ethyl)-2-(methylsulfinyl)-10H-phenothiazine; 10-(2-(1-Methyl-2-piperidyl)ethyl)-2-methylsulfinyl phenothiazine; 5588-33-0; C21H26N2OS2; CHEBI:6780; CHEMBL1088; HSDB 3357; NSC 186066; NSC186066; Tps23
|
| Indication |
Schizophrenia
[ICD11: 6A20]
|
Approved
|
[1]
|
| Structure |
|
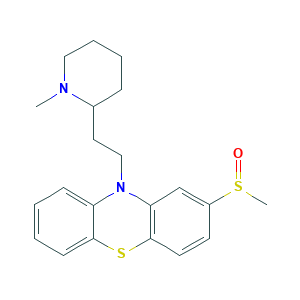 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
386.6 |
Topological Polar Surface Area |
68.1 |
| Heavy Atom Count |
26 |
Rotatable Bond Count |
4 |
| Hydrogen Bond Donor Count |
0 |
Hydrogen Bond Acceptor Count |
5 |
| Cross-matching ID |
- PubChem CID
- 4078
- PubChem SID
-
9352
; 447728
; 614172
; 4379956
; 7979909
; 8140528
; 8152557
; 10319751
; 10527260
; 11405940
; 11467677
; 11486174
; 14927472
; 17396840
; 26756521
; 29223187
; 46506724
; 47440446
; 47440447
; 47662489
; 47885582
; 48035319
; 48035320
; 48416224
; 49699103
; 50006596
; 50111387
; 50111388
; 50704712
; 57322129
; 85209561
; 85787941
; 85788345
; 92309158
; 96099906
; 103301322
; 104022598
; 104222090
; 104305362
; 124892406
; 125672983
; 129255022
; 134337566
; 134988360
; 137248555
; 139029518
; 144203603
; 144205571
; 160964272
; 163157019
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D04AAN
- Formula
- C21H26N2OS2
- Canonical SMILES
- CN1CCCCC1CCN2C3=CC=CC=C3SC4=C2C=C(C=C4)S(=O)C
- InChI
- 1S/C21H26N2OS2/c1-22-13-6-5-7-16(22)12-14-23-18-8-3-4-9-20(18)25-21-11-10-17(26(2)24)15-19(21)23/h3-4,8-11,15-16H,5-7,12-14H2,1-2H3
- InChIKey
- SLVMESMUVMCQIY-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


