Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR1084) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Miconazole
|
|||||
| Synonyms |
Miconazol; Miconazol [INN-Spanish]; Miconazole 3; Miconazole-7; Miconazolo; Miconazolo [DCIT]; Miconazolum; Miconazolum [INN-Latin]; Micozole; Minostate; Monazole 7; Monistat; Monistat 1 Combination Pack; Monistat IV; Monistat-Derm; R 18134; Brentan; Dactarin; Daktarin; Daktarin IV; Femizol-M; Florid(nitrate); MJR 1762; Vusion; Zimycan; miconazole; 1-(2-((2,4-Dichlorobenzyl)oxy)-2-(2,4-dichlorophenyl)ethyl)-1H-imidazole; 1-[2-(2,4-Dichlorophenyl)-2-[(2,4-dichlorophenyl)methoxy]ethyl]-1H-imidazole; 22916-47-8; C18H14Cl4N2O; NSC 170986
|
|||||
| Indication | Dermatophytosis [ICD11: 1F28] | Approved | [1] | |||
| Structure |
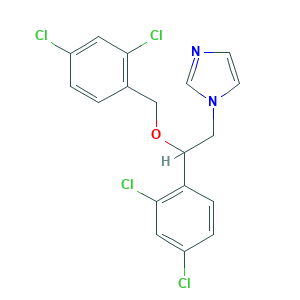 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 416.1 | Topological Polar Surface Area | 27 | ||
| Heavy Atom Count | 25 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 2 | |||
| Cross-matching ID |
|
|||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

