| General Information of Drug (ID:
DR1131) |
| Drug Name |
Naratriptan
|
| Synonyms |
Naratriptan; Naratriptanum; AMKVXSZCKVJAGH-UHFFFAOYSA-N; Colatan; QX3KXL1ZA2; 121679-13-8; 1H-Indole-5-ethanesulfonamide, N-methyl-3-(1-methyl-4-piperidinyl)-; CHEBI:7478; CHEMBL1278; N-Methyl-2-(3-(1-methylpiperidin-4-yl)-1H-indol-5-yl)ethanesulfonamide; N-methyl-2-(3-(1-methylpiperiden-4-yl)indole-5-yl)ethanesulfonamide; N-methyl-2-[3-(1-methyl-4-piperidyl)-1H-indol-5-yl]-ethanesulfonamide; N-methyl-2-[3-(1-methylpiperidin-4-yl)-1H-indol-5-yl]ethanesulfonamide; UNII-QX3KXL1ZA2
|
| Indication |
Migraine
[ICD11: 8A80]
|
Approved
|
[1]
|
| Structure |
|
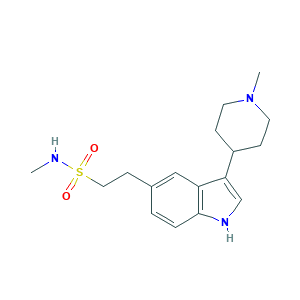 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
335.5 |
Topological Polar Surface Area |
73.6 |
| Heavy Atom Count |
23 |
Rotatable Bond Count |
5 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
4 |
| Cross-matching ID |
- PubChem CID
- 4440
- PubChem SID
-
9994
; 591979
; 4406062
; 7980085
; 8152740
; 14802133
; 29223537
; 46507243
; 46530540
; 48416313
; 49984092
; 50126335
; 50340505
; 57322272
; 85209198
; 92721601
; 93166470
; 96024943
; 103396287
; 103966995
; 104306415
; 118043420
; 124757290
; 125001918
; 125164094
; 125339313
; 126619011
; 126661482
; 126731399
; 127427940
; 134221895
; 134338167
; 135035417
; 135650691
; 135692164
; 137002440
; 139157587
; 143500462
; 144116266
; 152035273
; 152253555
; 152344169
; 160964291
; 164830541
; 174006312
; 175268422
; 176253695
; 179116807
; 196105283
; 210279333
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0T3KI
- Formula
- C17H25N3O2S
- Canonical SMILES
- CNS(=O)(=O)CCC1=CC2=C(C=C1)NC=C2C3CCN(CC3)C
- InChI
- 1S/C17H25N3O2S/c1-18-23(21,22)10-7-13-3-4-17-15(11-13)16(12-19-17)14-5-8-20(2)9-6-14/h3-4,11-12,14,18-19H,5-10H2,1-2H3
- InChIKey
- AMKVXSZCKVJAGH-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


