| General Information of Drug (ID:
DR1154) |
| Drug Name |
Niguldipine
|
| Synonyms |
Niguldipine [INN]; S(+)-niguldipine; SCHEMBL245992; Tocris-1123; Tocris-1124; Z81N45O25Z; ZINC100001967; niguldipine[inn]; (S)-Niguldipine; 113165-32-5; AC1L1TKT; AC1Q1ZUF; BDBM50034683; BDBM50453799; BRD-K59333713-003-01-2; CHEBI:103931; CHEMBL405355; CHEMBL41929; DB09239; DTXSID60274008; GTPL487; MFCD00873564; NCGC00025014-01; NCGC00025015-01; S(+)-1,4-Dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylic acid 3-(4,4-diphenyl-1-piperidinyl)-propyl methyl ester hydrochloride; UNII-Z81N45O25Z
|
| Indication |
Discovery agent
|
Investigative
|
[1]
|
| Structure |
|
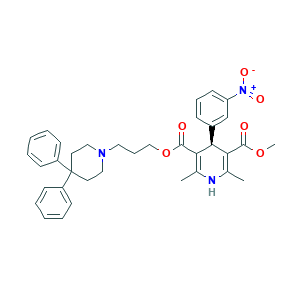 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
609.7 |
Topological Polar Surface Area |
114 |
| Heavy Atom Count |
45 |
Rotatable Bond Count |
11 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
8 |
| Cross-matching ID |
- PubChem CID
- 60602
- PubChem SID
-
8186958
; 11113931
; 11113932
; 14911982
; 43117981
; 80372166
; 85789015
; 103567485
; 103942848
; 104321194
; 117381126
; 134341334
; 135018553
; 135650717
; 139282946
; 179149614
; 184547327
; 198971262
; 225146125
; 225146175
; 226595902
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D05IML
- Formula
- C36H39N3O6
- Canonical SMILES
- CC1=C(C(C(=C(N1)C)C(=O)OCCCN2CCC(CC2)(C3=CC=CC=C3)C4=CC=CC=C4)C5=CC(=CC=C5)[N+](=O)[O-])C(=O)OC
- InChI
- 1S/C36H39N3O6/c1-25-31(34(40)44-3)33(27-12-10-17-30(24-27)39(42)43)32(26(2)37-25)35(41)45-23-11-20-38-21-18-36(19-22-38,28-13-6-4-7-14-28)29-15-8-5-9-16-29/h4-10,12-17,24,33,37H,11,18-23H2,1-3H3/t33-/m0/s1
- InChIKey
- SVJMLYUFVDMUHP-XIFFEERXSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


