| Synonyms |
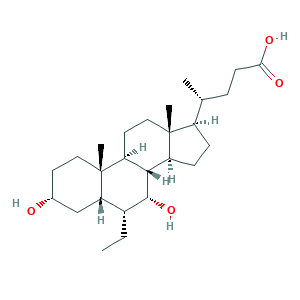
Obeticholic acid; Ocaliva; INT 747; INT-747; INT747; (4R)-4-[(3R,5S,6R,7R,8S,9S,10S,13R,14S,17R)-6-ethyl-3,7-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoic acid; 0462Z4S4OZ; 459789-99-2; 6-ECDCA; 6-ETHYL-CHENODEOXYCHOLIC ACID; 6-Ethyl-CDCA; 6-Ethylchenodeoxycholic acid; 6ECDCA; 6alpha-Ethyl-chenodeoxycholic acid; 6alpha-ethylchenodeoxycholic acid; DSP-1747; UNII-0462Z4S4OZ
|
| Cross-matching ID |
- PubChem CID
- 447715
- PubChem SID
-
828938
; 828951
; 7886586
; 7888489
; 10300105
; 14880306
; 16101459
; 17396626
; 36889785
; 46392814
; 49700782
; 53789454
; 57404826
; 76486275
; 96026040
; 103700141
; 104638392
; 123055342
; 126680398
; 135263637
; 163122223
; 175265866
; 176236560
; 178100437
; 204383201
; 219813737
; 223670674
; 224721644
; 227005340
; 251970994
; 252300974
; 252553933
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0M4WA
- Formula
- C26H44O4
- Canonical SMILES
- CCC1C2CC(CCC2(C3CCC4(C(C3C1O)CCC4C(C)CCC(=O)O)C)C)O
- InChI
- 1S/C26H44O4/c1-5-17-21-14-16(27)10-12-26(21,4)20-11-13-25(3)18(15(2)6-9-22(28)29)7-8-19(25)23(20)24(17)30/h15-21,23-24,27,30H,5-14H2,1-4H3,(H,28,29)/t15-,16-,17-,18-,19+,20+,21+,23+,24-,25-,26-/m1/s1
- InChIKey
- ZXERDUOLZKYMJM-ZWECCWDJSA-N
|