| General Information of Drug (ID:
DR1216) |
| Drug Name |
CCRIS-4201
|
| Synonyms |
Coretal; Osprenololo [DCIT]; Oxprenolol [INN:BAN]; Oxprenolol, dl-; Oxprenololum; Oxprenololum [INN-Latin]; Trasacor; Trasicor; dl-Oxprenolol; oxprenolol; (+)-1-(o-(Allyloxy)phenoxy)-3-(isopropylamino)propan-2-ol; (+-)-Oxprenolol; (1)-1-(o-(Allyloxy)phenoxy)-3-(isopropylamino)propan-2-ol; 1-(Isopropylamino)-2-hydroxy-3-(o-(allyloxy)phenoxy)propane; 1-(o-(Allyloxy)phenoxy)-3-(isopropylamino)-2-propanol; 6452-71-7; CCRIS 4201; CHEMBL546; EINECS 229-257-9; EINECS 245-359-6
|
| Indication |
Essential hypertension
[ICD11: BA00]
|
Phase 1
|
[1]
|
| Structure |
|
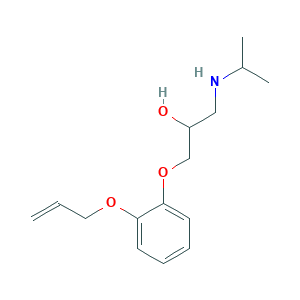 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
265.35 |
Topological Polar Surface Area |
50.7 |
| Heavy Atom Count |
19 |
Rotatable Bond Count |
9 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
4 |
| Cross-matching ID |
- PubChem CID
- 4631
- PubChem SID
-
597348
; 5204100
; 7980224
; 8152843
; 10531628
; 11467085
; 11468205
; 11486673
; 14750338
; 29223720
; 46508996
; 47217074
; 47365481
; 47885676
; 48110749
; 49699389
; 50022613
; 50195457
; 57322363
; 81065509
; 85220236
; 85788907
; 96025005
; 103176474
; 104098253
; 104306967
; 126428554
; 127613395
; 134337778
; 134988108
; 135048217
; 135048218
; 137111941
; 142640337
; 160964820
; 163228832
; 178103829
; 179150852
; 224791627
; 226406507
; 241041980
; 241128481
; 249934544
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0X2MB
- Formula
- C15H23NO3
- Canonical SMILES
- CC(C)NCC(COC1=CC=CC=C1OCC=C)O
- InChI
- 1S/C15H23NO3/c1-4-9-18-14-7-5-6-8-15(14)19-11-13(17)10-16-12(2)3/h4-8,12-13,16-17H,1,9-11H2,2-3H3
- InChIKey
- CEMAWMOMDPGJMB-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


