| General Information of Drug (ID:
DR1318) |
| Drug Name |
Practolol
|
| Synonyms |
Practololo [DCIT]; Practololum; Practololum [INN-Latin]; Praktololu; Praktololu [Polish]; Teranol; dl-Practolol; practolol; rac Practolol; Dalzic; Eraldin; Eralzdin Practolol; 1-(4-Acetamidophenoxy)-3-isopropylamino-2-propanol; 4'-(2-Hydroxy-3-(isopropylamino)propoxy)acetanilide; 6673-35-4; AY 21011; CCRIS 1089; ICI 50172; ICI-50172; N-(4-(2-Hydroxy-3-((1-methylethyl)amino)propoxy)phenyl)acetamide; N-[4-[2-HYDROXY-3-[(1-METHYLETHYL)AMINO]PROPOXY]PHENYL]ACETAMIDE; N-{4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl}acetamide
|
| Indication |
Cardiac arrhythmia
[ICD11: BC65]
|
Phase 4
|
[1]
|
| Structure |
|
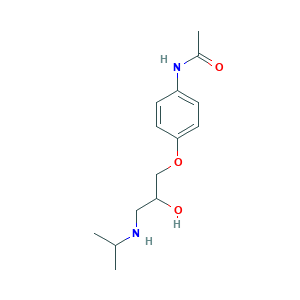 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
266.34 |
Topological Polar Surface Area |
70.6 |
| Heavy Atom Count |
19 |
Rotatable Bond Count |
7 |
| Hydrogen Bond Donor Count |
3 |
Hydrogen Bond Acceptor Count |
4 |
| Cross-matching ID |
- PubChem CID
- 4883
- PubChem SID
-
13861
; 4500647
; 7979385
; 8150016
; 8153002
; 10321694
; 11336076
; 11361315
; 11364348
; 11366910
; 11369472
; 11374635
; 11377634
; 11462287
; 11466360
; 11467480
; 11485240
; 11486020
; 11489388
; 11492674
; 11495268
; 15197223
; 26679880
; 26751910
; 26980600
; 29223962
; 46507496
; 47207250
; 47440331
; 47959823
; 48185058
; 48259303
; 48334570
; 48334571
; 48334572
; 48414361
; 49698824
; 49860734
; 50104588
; 56413037
; 56463034
; 57322502
; 80701653
; 85209244
; 85451356
; 85788851
; 87660772
; 92125329
; 92308845
; 99301989
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0KD1U
- Formula
- C14H22N2O3
- Canonical SMILES
- CC(C)NCC(COC1=CC=C(C=C1)NC(=O)C)O
- InChI
- 1S/C14H22N2O3/c1-10(2)15-8-13(18)9-19-14-6-4-12(5-7-14)16-11(3)17/h4-7,10,13,15,18H,8-9H2,1-3H3,(H,16,17)
- InChIKey
- DURULFYMVIFBIR-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


