| Synonyms |
Pralatrexate; Pralatrexate(Folotyn); (2S)-2-((4-((1RS)-1-((2,4-Diaminopteridin-6-yl)methyl)but-3-ynyl)benzoyl)amino)pentanedioic acid; Folotyn; (2S)-2-(4-(1-(2,4-diaminopteridin-6-yl)pent-4-yn-2-yl)benzamido)pentanedioic acid; 10-Propargyl-10-deazaaminopterin; 146464-95-1; CHEBI:71223; HSDB 7786; N-(4-(1-((2,4-Diamino-6-pteridinyl)methyl)-3-butynyl)benzoyl)-L-glutamic acid; N-[4-[1-[(2,4-Diamino-6-pteridinyl)methyl]-3-butyn-1-yl]benzoyl]-L-glutamic Acid; NCGC00242596-01; PDX
|
| Cross-matching ID |
- PubChem CID
- 148121
- PubChem SID
-
10249810
; 14761163
; 14907808
; 46225076
; 47207252
; 57346701
; 76049103
; 99437279
; 103771482
; 104418267
; 124950714
; 126666101
; 131408688
; 134223179
; 135692165
; 136367814
; 137248707
; 141792755
; 144206459
; 144236486
; 152106310
; 152258219
; 152344466
; 160646081
; 160647055
; 164044033
; 164232763
; 164764489
; 175427094
; 175437712
; 176484650
; 178103446
; 180191813
; 184527271
; 186014781
; 198993109
; 223392464
; 223659651
; 223702389
; 225255488
; 226410872
; 240035409
; 241036657
; 243124404
; 249868098
; 252069088
; 252214139
; 252473148
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D02LWU
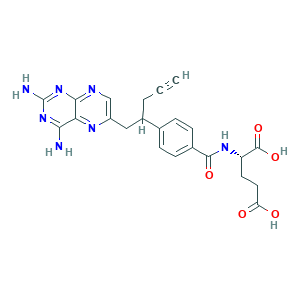
- Formula
- C23H23N7O5
- Canonical SMILES
- C#CCC(CC1=CN=C2C(=N1)C(=NC(=N2)N)N)C3=CC=C(C=C3)C(=O)NC(CCC(=O)O)C(=O)O
- InChI
- 1S/C23H23N7O5/c1-2-3-14(10-15-11-26-20-18(27-15)19(24)29-23(25)30-20)12-4-6-13(7-5-12)21(33)28-16(22(34)35)8-9-17(31)32/h1,4-7,11,14,16H,3,8-10H2,(H,28,33)(H,31,32)(H,34,35)(H4,24,25,26,29,30)/t14?,16-/m0/s1
- InChIKey
- OGSBUKJUDHAQEA-WMCAAGNKSA-N
|