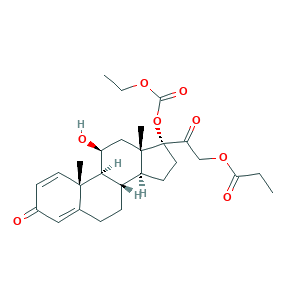
| Synonyms |
Peitel; Prednicarbat; Prednicarbate (USP/INN); Prednicarbato; Prednicarbato [INN-Spanish]; Prednicarbatum; Prednicarbatum [INN-Latin]; Prednitop; Regenit; Dermatop; Dermatop (TN); Dermatop E emollient; PREDNICARBATE; S 770777; S-770777; V901LV1K7D; 73771-04-7; EINECS 277-590-3; Hoe 777; Hoe-777; MLS002154121; UNII-V901LV1K7D; [2-[(8S,9S,10R,11S,13S,14S,17R)-17-ethoxycarbonyloxy-11-hydroxy-10,13-dimethyl-3-oxo-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl] propanoate
|
| Cross-matching ID |
- PubChem CID
- 6714002
- PubChem SID
-
11467072
; 11468192
; 11486640
; 12013808
; 14810426
; 17190443
; 43333414
; 47207264
; 47574670
; 47871009
; 47945107
; 48244692
; 49699264
; 50124068
; 50655143
; 56463553
; 57371370
; 71825010
; 85787547
; 92125934
; 92720065
; 103770369
; 103914660
; 114951144
; 121363605
; 124799601
; 126620396
; 126651370
; 134223290
; 135012724
; 137240385
; 144204238
; 163835804
; 164232786
; 164814618
; 175267150
; 175612124
; 179149412
; 196105692
; 223365940
; 223680208
; 226396028
; 252356176
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D09IEE
- Formula
- C27H36O8
- Canonical SMILES
- CCC(=O)OCC(=O)C1(CCC2C1(CC(C3C2CCC4=CC(=O)C=CC34C)O)C)OC(=O)OCC
- InChI
- 1S/C27H36O8/c1-5-22(31)34-15-21(30)27(35-24(32)33-6-2)12-10-19-18-8-7-16-13-17(28)9-11-25(16,3)23(18)20(29)14-26(19,27)4/h9,11,13,18-20,23,29H,5-8,10,12,14-15H2,1-4H3/t18-,19-,20-,23+,25-,26-,27-/m0/s1
- InChIKey
- FNPXMHRZILFCKX-KAJVQRHHSA-N
|