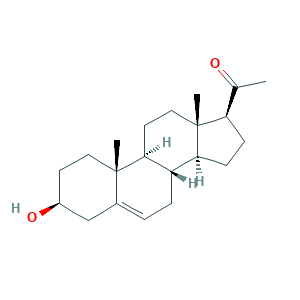
| Synonyms |
Pregnenolona [INN-Spanish]; Pregnenolone [INN:BAN]; Pregnenolonum [INN-Latin]; Pregnetan; Pregneton; Pregnolon; Prenolon; Prestwick3_000546; Arthenolone; Enelone; Natolone; Prestwick_859; Regnosone; Skinostelon; delta5-Pregnenolone; pregnenolone; (3BETA)-3-HYDROXYPREGN-5-EN-20-ONE; 145-13-1; 3-beta-Hydroxypregn-5-en-20-one; 3beta-Hydroxy-5-pregnen-20-one; 3beta-Hydroxypregn-5-en-20-one; 5-Pregnen-3-beta-ol-20-one; 5-Pregnen-3beta-ol-20-one; 5-Pregnenolone; Bina-Skin; NSC 1616; Pregn-5-en-20-one, 3-hydroxy-, (3beta)-; UNII-73R90F7MQ8
|
| Cross-matching ID |
- PubChem CID
- 8955
- PubChem SID
-
3095
; 5056
; 841801
; 3136425
; 7847211
; 7889910
; 8143499
; 8156371
; 10321800
; 11466574
; 11467694
; 11486248
; 12157331
; 14850333
; 24702313
; 24887880
; 24899008
; 25621392
; 29227585
; 46506718
; 47275669
; 47646662
; 48094643
; 48318548
; 48394039
; 49698547
; 50019742
; 50111369
; 50952978
; 53790378
; 56463239
; 57325339
; 85165262
; 87574922
; 90451278
; 92125557
; 92298073
; 92309191
; 92712002
; 93167037
; 93576762
; 103557063
; 103845983
; 103914285
; 104319171
; 121363228
; 124812051
; 124892396
; 126630318
; 126656995
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0B4RU
- Formula
- C21H32O2
- Canonical SMILES
- CC(=O)C1CCC2C1(CCC3C2CC=C4C3(CCC(C4)O)C)C
- InChI
- 1S/C21H32O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h4,15-19,23H,5-12H2,1-3H3/t15-,16-,17+,18-,19-,20-,21+/m0/s1
- InChIKey
- ORNBQBCIOKFOEO-QGVNFLHTSA-N
|