Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR1415) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Ribociclib succinate
|
|||||
| Synonyms |
Ribociclib succinate; Ribociclib succinate (USAN); Ribociclib succinate [USAN]; UNII-BG7HLX2919; 1374639-75-4; 7-Cyclopentyl-N,N-dimethyl-2-((5-(piperazin-1-yl)pyridin-2-yl)amino)-7H-pyrrolo[2,3-d]pyrimidine-6-carboxamide succinate; BG7HLX2919; Butanedioic acid, compd. with 7-cyclopentyl-N,N-dimethyl-2-((5-(1-piperazinyl)-2-pyridinyl)amino)-7H-pyrrolo(2,3-d)pyrimidine-6-carboxamide (1:1); LEE-011 succinate; LEE011 succinate; LEE011-BBA
|
|||||
| Indication | Breast cancer [ICD11: 2C60] | Approved | [1] | |||
| Structure |
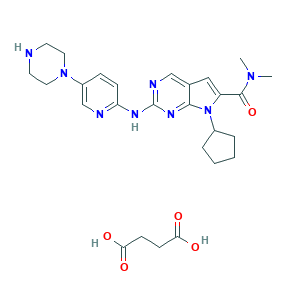 |
|||||
| 3D MOL is unavailable | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 552.6 | Topological Polar Surface Area | 166 | ||
| Heavy Atom Count | 40 | Rotatable Bond Count | 8 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 11 | |||
| Cross-matching ID |
|
|||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

