| Synonyms |
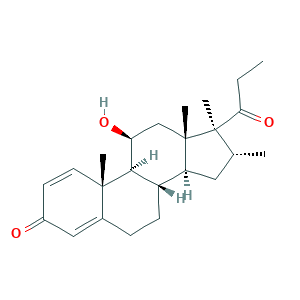
Rimexolon; Rimexolona; O7M2E4264D; Org 6216; Org-6216; RIMEXOLONE; Rimexel; Rimexolona [INN-Spanish]; Rimexolone [USAN:USP:INN:BAN]; Rimexolonum; Rimexolonum [INN-Latin]; Trimexolone; (8S,9S,10R,11S,13S,14S,16R,17S)-11-hydroxy-10,13,16,17-tetramethyl-17-propanoyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-3-one; 11beta-Hydroxy-16alpha,17,21-trimethyl-1,4-pregnadien-3,20-dion; 11beta-Hydroxy-16alpha,17alpha-dimethyl-17-propionylandrosta-1,4-dien-3-one; 49697-38-3; AL 02178; MLS002154105; UNII-O7M2E4264D; Vexol
|
| Cross-matching ID |
- PubChem CID
- 5311412
- PubChem SID
-
7980520
; 11056486
; 11467048
; 11468168
; 11486786
; 12014133
; 14804034
; 14828819
; 39341090
; 47207390
; 47871005
; 47871006
; 47871007
; 48170707
; 49699253
; 50123981
; 56463550
; 57359468
; 80240860
; 85788089
; 92126045
; 103770600
; 103914672
; 114156025
; 121363716
; 124801082
; 134224655
; 135000880
; 137005248
; 141792894
; 164233438
; 164764869
; 175267976
; 175612108
; 178103677
; 179149417
; 226767286
; 244890192
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0D2TN
- Formula
- C24H34O3
- Canonical SMILES
- CCC(=O)C1(C(CC2C1(CC(C3C2CCC4=CC(=O)C=CC34C)O)C)C)C
- InChI
- 1S/C24H34O3/c1-6-20(27)24(5)14(2)11-18-17-8-7-15-12-16(25)9-10-22(15,3)21(17)19(26)13-23(18,24)4/h9-10,12,14,17-19,21,26H,6-8,11,13H2,1-5H3/t14-,17+,18+,19+,21-,22+,23+,24-/m1/s1
- InChIKey
- QTTRZHGPGKRAFB-OOKHYKNYSA-N
|