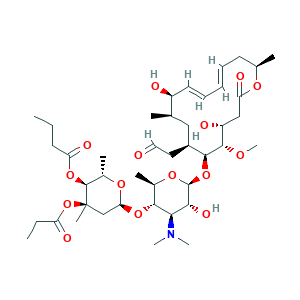
| Cross-matching ID |
- PubChem CID
- 5282211
- PubChem SID
-
7848959
; 8616945
; 12012977
; 14865109
; 39315691
; 48416480
; 49988909
; 57358575
; 79070251
; 113856681
; 117623998
; 124766125
; 126666648
; 135029394
; 136923440
; 137102255
; 162224733
; 163414148
; 163849054
; 179151058
; 184546358
; 189053409
; 196379320
; 198948647
; 223680285
; 226504009
; 241133675
; 252356495
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0L6QI
- Formula
- C42H69NO15
- Canonical SMILES
- CCCC(=O)OC1C(OC(CC1(C)OC(=O)CC)OC2C(OC(C(C2N(C)C)O)OC3C(CC(C(C=CC=CCC(OC(=O)CC(C3OC)O)C)O)C)CC=O)C)C
- InChI
- 1S/C42H69NO15/c1-11-16-32(48)55-40-27(6)53-34(23-42(40,7)58-31(47)12-2)56-37-26(5)54-41(36(50)35(37)43(8)9)57-38-28(19-20-44)21-24(3)29(45)18-15-13-14-17-25(4)52-33(49)22-30(46)39(38)51-10/h13-15,18,20,24-30,34-41,45-46,50H,11-12,16-17,19,21-23H2,1-10H3/b14-13+,18-15+/t24-,25-,26-,27+,28+,29+,30-,34+,35-,36-,37-,38+,39+,40+,41+,42-/m1/s1
- InChIKey
- VYWWNRMSAPEJLS-MDWYKHENSA-N
|