| Cross-matching ID |
- PubChem CID
- 68770
- PubChem SID
-
8192333
; 14926104
; 26757961
; 43125295
; 50060575
; 50068853
; 51091522
; 57317059
; 77170990
; 95612779
; 98940985
; 103096738
; 103401016
; 104343015
; 107929351
; 117537352
; 124580994
; 125712702
; 126580194
; 126725781
; 126737449
; 127339737
; 127339738
; 127339739
; 131492773
; 135027207
; 135066580
; 135555209
; 135653641
; 135707467
; 135888304
; 136895027
; 137114987
; 142640331
; 144205724
; 162057748
; 162118068
; 162848586
; 164765202
; 165745358
; 170466514
; 170558731
; 178101379
; 179323274
; 184547412
; 202554924
; 215742084
; 226457382
; 252091637
; 252492541
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D05JKG
- Formula
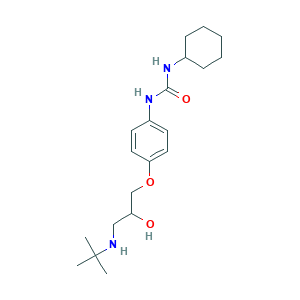
- C20H33N3O3
- Canonical SMILES
- CC(C)(C)NCC(COC1=CC=C(C=C1)NC(=O)NC2CCCCC2)O
- InChI
- 1S/C20H33N3O3/c1-20(2,3)21-13-17(24)14-26-18-11-9-16(10-12-18)23-19(25)22-15-7-5-4-6-8-15/h9-12,15,17,21,24H,4-8,13-14H2,1-3H3,(H2,22,23,25)
- InChIKey
- MXFWWQICDIZSOA-UHFFFAOYSA-N
|