| Synonyms |
ADXGNEYLLLSOAR-UHFFFAOYSA-N; PubChem24430; TASOSARTAN; Tasosartan (USAN/INN); Tasosartan [USAN:INN:BAN]; Verdia; WAY-126756; WAY-ANA-756; 145733-36-4; 2,4-dimethyl-8-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]-5,6-dihydropyrido[2,3-d]pyrimidin-7-one; 2,4-dimethyl-8-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]-5,6-dihydropyrido[6,5-d]pyrimidin-7-one; 48G92V856H; AC1L1U5X; AC1Q6LAA; ACT06754; ANA-756; BCP06091; CHEBI:135666; CHEMBL432162; DTXSID40163148; EX-A082; GTPL6898; HY-A0250; KS-000000TK; SCHEMBL49626; UNII-48G92V856H
|
| Cross-matching ID |
- PubChem CID
- 60919
- PubChem SID
-
12014735
; 14781918
; 43118241
; 46504809
; 47207668
; 50241161
; 57314180
; 85210063
; 103271083
; 103972483
; 104253198
; 104321954
; 104826537
; 117600003
; 124757326
; 125164130
; 125823197
; 126681832
; 127392389
; 134338495
; 135016861
; 137185590
; 139561026
; 140917228
; 141045621
; 143493280
; 152233049
; 152343908
; 152344365
; 160964640
; 162011534
; 163418539
; 163849994
; 164814968
; 165238016
; 172919626
; 174527556
; 178103480
; 179149947
; 179323284
; 184545958
; 198987382
; 223659560
; 224992574
; 225380894
; 226433336
; 241036477
; 242060035
; 244153220
; 249582367
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0M7TO
- Formula
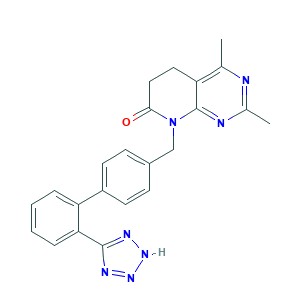
- C23H21N7O
- Canonical SMILES
- CC1=C2CCC(=O)N(C2=NC(=N1)C)CC3=CC=C(C=C3)C4=CC=CC=C4C5=NNN=N5
- InChI
- 1S/C23H21N7O/c1-14-18-11-12-21(31)30(23(18)25-15(2)24-14)13-16-7-9-17(10-8-16)19-5-3-4-6-20(19)22-26-28-29-27-22/h3-10H,11-13H2,1-2H3,(H,26,27,28,29)
- InChIKey
- ADXGNEYLLLSOAR-UHFFFAOYSA-N
|