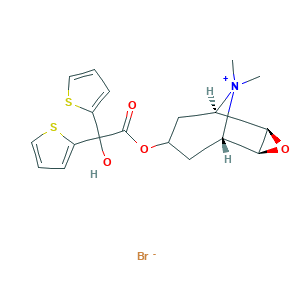
| Synonyms |
Tiopropium; Tiotropium (Bromide); UNII-XX112XZP0J; XX112XZP0J; (1R,2R,4S,5S,7S)-7-(2-Hydroxy-2,2-di(thiophen-2-yl)acetoxy)-9,9-dimethyl-3-oxa-9-azatricyclo[3.3.1.0; (1R,2R,4S,5S,7s)-7-(2-Hydroxy-2,2-di(thiophen-2-yl)acetoxy)-9,9-dimethyl-3-oxa-9-azatricyclo[3.3.1.02,4]nonan-9-ium bromide; 136310-93-5; BA 679BR; Spiriva Handihaler; Spiriva Respimat; TIOTROPIUM BROMIDE
|
| Cross-matching ID |
- PubChem CID
- 5487426
- PubChem SID
-
12014767
; 16431254
; 16516650
; 37101841
; 57364284
; 78006594
; 92309156
; 92714242
; 93302582
; 121264529
; 125003137
; 126592377
; 126624303
; 126655544
; 134224129
; 134337655
; 135087660
; 135693784
; 136376471
; 136946674
; 144206151
; 152059683
; 152258759
; 152343784
; 160647604
; 162038024
; 162170559
; 164194996
; 164814954
; 172440019
; 172440020
; 172919722
; 174476476
; 175268305
; 180673829
; 184811782
; 187072489
; 196106129
; 198991441
; 223658400
; 226399777
; 226763578
; 233206887
; 241032671
; 242592071
; 249845981
; 250203609
; 251916428
; 251917775
; 252039050
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0Z7AB
- Formula
- C19H22BrNO4S2
- Canonical SMILES
- C[N+]1(C2CC(CC1C3C2O3)OC(=O)C(C4=CC=CS4)(C5=CC=CS5)O)C.[Br-]
- InChI
- 1S/C19H22NO4S2.BrH/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15;/h3-8,11-13,16-17,22H,9-10H2,1-2H3;1H/q+1;/p-1/t11?,12-,13+,16-,17+;
- InChIKey
- DQHNAVOVODVIMG-RGECMCKFSA-M
|