| DM Name |
DM ID |
PubChem ID |
Reaction |
DM Level |
REF |
|
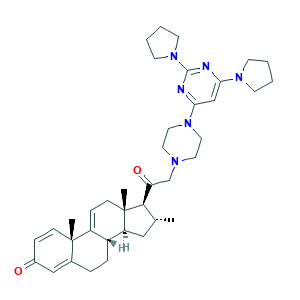
(6S,8S,10R,13S,14S,16R,17S)-17-[2-[4-(2,6-dipyrrolidin-1-ylpyrimidin-4-yl)piperazin-1-yl]acetyl]-6-hydroxy-10,13,16-trimethyl-6,7,8,12,14,15,16,17-octahydrocyclopenta[a]phenanthren-3-one
|
DM005751
|
|
Oxidation
-
Hydrolyzationn |
1 |
[2]
|
|
17-[2-[4-(2,6-Dipyrrolidin-1-ylpyrimidin-4-yl)piperazin-1-yl]acetyl]-6-hydroxy-10,13,16-trimethyl-6,7,8,12,14,15,16,17-octahydrocyclopenta[a]phenanthren-3-one
|
DM005752
|
|
Oxidation
-
Hydrolyzationn |
1 |
[2]
|
|
17-[2-[4-[2-(3-Hydroxypyrrolidin-1-yl)-6-pyrrolidin-1-ylpyrimidin-4-yl]piperazin-1-yl]acetyl]-10,13,16-trimethyl-6,7,8,12,14,15,16,17-octahydrocyclopenta[a]phenanthren-3-one
|
DM005753
|
|
Oxidation
-
Hydrolyzationn |
1 |
[2]
|
|
17-[2-[4-[6-(3-Hydroxypyrrolidin-1-yl)-2-pyrrolidin-1-ylpyrimidin-4-yl]piperazin-1-yl]acetyl]-10,13,16-trimethyl-6,7,8,12,14,15,16,17-octahydrocyclopenta[a]phenanthren-3-one
|
DM005754
|
|
Oxidation
-
Hydrolyzationn |
1 |
[2]
|
|
|
|
|
|
|
|