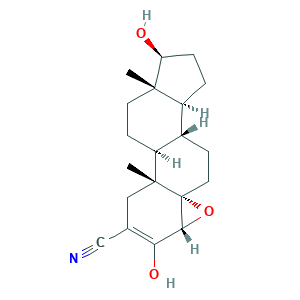
| Synonyms |
Trilostane; Trilostano; Trilostanum; Vetoryl; WIN 24,540; Win-24540; Desopan; L0FPV48Q5R; Modrastane; Modrenal; (1aR,4aR,4bS,6aS,7S,9aS,9bS,11aS)-2,7-dihydroxy-4a,6a-dimethyl-1a,4,4a,4b,5,6,6a,7,8,9,9a,9b,10,11-tetradecahydrocyclopenta[7,8]phenanthro[1,10a-b]oxirene-3-carbonitrile; (4alpha,5alpha,17beta)-3,17-dihydroxy-4,5-epoxyandrost-2-ene-2-carbonitrile; 13647-35-3; CHEBI:32260; DSSTox_CID_3706; DSSTox_GSID_23706; DSSTox_RID_77157; MFCD00199295; UNII-L0FPV48Q5R
|
| Cross-matching ID |
- PubChem CID
- 656583
- PubChem SID
-
582970
; 7848243
; 9268061
; 14753126
; 14850960
; 46507062
; 48416665
; 53789389
; 56352900
; 57408348
; 77191778
; 92716671
; 99437135
; 103770890
; 113527831
; 124757227
; 125164031
; 126591224
; 126622687
; 126654263
; 127324070
; 127324071
; 127324072
; 127324073
; 127324074
; 134337470
; 134990249
; 135692137
; 136946482
; 137005257
; 143492476
; 144206007
; 144212723
; 152103462
; 152164604
; 152242918
; 160964442
; 162170394
; 163138502
; 164194095
; 165702349
; 170465836
; 175265964
; 176262060
; 176484985
; 178103456
; 179149866
; 196105623
; 210275073
; 210280711
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D03XOC
- Formula
- C20H27NO3
- Canonical SMILES
- CC12CCC3C(C1CCC2O)CCC45C3(CC(=C(C4O5)O)C#N)C
- InChI
- 1S/C20H27NO3/c1-18-7-6-14-12(13(18)3-4-15(18)22)5-8-20-17(24-20)16(23)11(10-21)9-19(14,20)2/h12-15,17,22-23H,3-9H2,1-2H3/t12-,13-,14-,15-,17+,18-,19+,20+/m0/s1
- InChIKey
- KVJXBPDAXMEYOA-CXANFOAXSA-N
|