| General Information of Drug (ID:
DR1684) |
| Drug Name |
Vilanterol
|
| Synonyms |
Vilanterol; Vilanterol (USAN); Vilanterol [USAN:INN]; Vilanterol base; ZINC3991624; vilanterolum; 028LZY775B; 4-((1R)-2-((6-(2-((2,6-dichlorophenyl)methoxy)ethoxy)hexyl)amino)-1-hydroxyethyl)-2-(hydroxymethyl)phenol; 4-[(1R)-2-[(6-{2-[(2,6-dichlorophenyl)methoxy]ethoxy}hexyl)amino]-1-hydroxyethyl]-2-(hydroxymethyl)phenol; DAFYYTQWSAWIGS-DEOSSOPVSA-N; SCHEMBL142630; 503068-34-6; BCP25897; CHEBI:75037; CHEMBL1198857; DTXSID80198318; EX-A2177; GTPL7353; GW 642444; GW 642444X; GW-642444x; GW642444x; MFCD18782703; UNII-028LZY775B
|
| Indication |
Asthma
[ICD11: CA23]
|
Approved
|
[1]
|
| Structure |
|
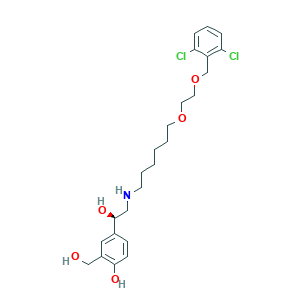 |
|
3D MOL is unavailable
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
486.4 |
Topological Polar Surface Area |
91.2 |
| Heavy Atom Count |
32 |
Rotatable Bond Count |
16 |
| Hydrogen Bond Donor Count |
4 |
Hydrogen Bond Acceptor Count |
6 |
| Cross-matching ID |
- PubChem CID
- 10184665
- PubChem SID
-
15180153
; 22579535
; 40274235
; 80028218
; 124490436
; 135291438
; 137063375
; 160677153
; 163365084
; 163835568
; 175268108
; 178103925
; 210275079
; 210280717
; 223936788
; 226509475
; 242700176
; 250184675
; 251963097
; 252224058
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0L0GM
- Formula
- C24H33Cl2NO5
- Canonical SMILES
- C1=CC(=C(C(=C1)Cl)COCCOCCCCCCNCC(C2=CC(=C(C=C2)O)CO)O)Cl
- InChI
- 1S/C24H33Cl2NO5/c25-21-6-5-7-22(26)20(21)17-32-13-12-31-11-4-2-1-3-10-27-15-24(30)18-8-9-23(29)19(14-18)16-28/h5-9,14,24,27-30H,1-4,10-13,15-17H2/t24-/m0/s1
- InChIKey
- DAFYYTQWSAWIGS-DEOSSOPVSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


