| Synonyms |
Vigantol; Vigorsan; Vitamin D3; Vitinc Dan-Dee-3; cholecalciferol; vitamin d-3; (+)-Vitamin D3; Activated 7-dehydrocholesterol; Arachitol; Calciol; Cholecalciferol, D3; Cholecalciferolum; Colecalciferol; Colecalciferolo [DCIT]; Colecalciferolum; Colecalciferolum [INN-Latin]; Colecalcipherol; D3-Vicotrat; D3-Vigantol; Delsterol; Deparal; Duphafral D3 1000; Ebivit; FeraCol; NEO Dohyfral D3; Oleovitamin D3; Quintox; Rampage; Ricketon; Trivitan; VITAMIN D; Vi-De3; Vi-de-3-hydrosol; 1406-16-2; 67-97-0; 7-Dehydrocholestrol, activated;; MFCD00078131
|
| Cross-matching ID |
- PubChem CID
- 5280795
- ChEBI ID
-
- CAS Number
-
- Formula
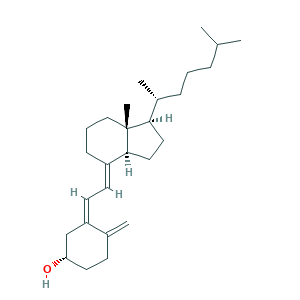
- C27H44O
- Canonical SMILES
- CC(C)CCCC(C)C1CCC2C1(CCCC2=CC=C3CC(CCC3=C)O)C
- InChI
- 1S/C27H44O/c1-19(2)8-6-9-21(4)25-15-16-26-22(10-7-17-27(25,26)5)12-13-23-18-24(28)14-11-20(23)3/h12-13,19,21,24-26,28H,3,6-11,14-18H2,1-2,4-5H3/b22-12+,23-13-/t21-,24+,25-,26+,27-/m1/s1
- InChIKey
- QYSXJUFSXHHAJI-YRZJJWOYSA-N
|