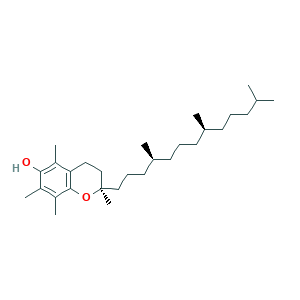
| Synonyms |
Almefrol; Antisterility vitamin; Vitamin E; Covi-ox; Covitol F 1000; Denamone; E-Vimin; Emipherol; Endo E; Eprolin S; Epsilan; Etamican; Etavit; Evitaminum; Ilitia; Profecundin; Rhenogran Ronotec 50; Spavit E; Syntopherol; Tenox GT 1; Tokopharm; VITAMIN E; Vascuals; Verrol; Viprimol; Vita E; Vitamin E alpha; Vitaplex E; Vitayonon; Viteolin; Viterra E; alpha Tocopherol; alpha-Tocopherol acid; alpha-Tokoferol; (R)-2,5,7,8-tetramethyl-2-((4S,8S)-4,8,12-trimethyltridecyl)chroman-6-ol; 77171-98-3; E 307 (tocopherol); E Prolin; Esorb; Evion; Lan-E; Med-E; Vi-E
|
| Cross-matching ID |
- PubChem CID
- 6560141
- CAS Number
-
- TTD Drug ID
- D02TQO
- Formula
- C29H50O2
- Canonical SMILES
- CC1=C(C2=C(CCC(O2)(C)CCCC(C)CCCC(C)CCCC(C)C)C(=C1O)C)C
- InChI
- 1S/C29H50O2/c1-20(2)12-9-13-21(3)14-10-15-22(4)16-11-18-29(8)19-17-26-25(7)27(30)23(5)24(6)28(26)31-29/h20-22,30H,9-19H2,1-8H3/t21-,22-,29-/m1/s1
- InChIKey
- GVJHHUAWPYXKBD-IHMCZWCLSA-N
|