Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR1728) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Ziconotide
|
|||||
| Synonyms |
Conotoxin MVIIA; Conotoxin MVIIA_Smartox; Omega conotoxin MVIIA; Prialt; SNX-111; Ziconotide; Ziconotide [USAN:INN]; o-Conotoxin MVIIA; omega-Conopeptide MVIIA (Conus); omega-Conotoxin M VIIA; omega-conotoxin MVIIA; 08CON001; 107452-89-1; CHEBI:135912; CHEMBL2103752; CTX MVIIA; DRG-0250; DTXSID60883174; GTPL2536; HSDB 7609; MFCD00145036; UNII-7I64C51O16; omega-Conotoxin M VIIA (reduced), cyclic (1-16),(8-20),(15-25)-tris(disulfide); omega-Conotoxin mviia, conus magus
|
|||||
| Indication | Anaesthesia [ICD11: 8E22] | Approved | [1] | |||
| Structure |
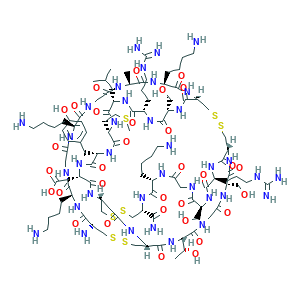 |
|||||
| 3D MOL is unavailable | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 2639.2 | Topological Polar Surface Area | 1310 | ||
| Heavy Atom Count | 177 | Rotatable Bond Count | 40 | |||
| Hydrogen Bond Donor Count | 42 | Hydrogen Bond Acceptor Count | 46 | |||
| Cross-matching ID |
|
|||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

