| Synonyms |
Glimid; Glutarimide, 2-ethyl-2-phenyl-; Glutathimid; Glutethimid; Glutethimidum; Glutetimid; Glutetimide; Glutetimide [DCIT]; Glutetimidu; Glutetimidu [Polish]; Gluthetimide; Noxiron; Noxyron; Ondasil; Rigenox; Sarodormin; Alfimid; Doriden; Doriden-sed; Dorimide; Elrodorm; dl-Glutethimide; glutethimide; 2-Ethyl-2-phenylglutarimide; 2-Phenyl-2-ethylglutaric acid imide; 3-Ethyl-3-phenyl-2,6-dioxopiperidine; 3-Ethyl-3-phenyl-2,6-piperidinedione; 3-Phenyl-3-ethyl-2,6-diketopiperidine; 3-Phenyl-3-ethyl-2,6-dioxopiperidine; 77-21-4; Gimid
|
| Cross-matching ID |
- PubChem CID
- 3487
- PubChem SID
-
9692
; 400159
; 5047947
; 7847598
; 8152215
; 10535494
; 15414774
; 26754495
; 29215340
; 29222619
; 46506283
; 48416061
; 49973115
; 53786842
; 57321835
; 78011658
; 83568923
; 92714692
; 103304476
; 104303656
; 117423004
; 124892162
; 125357548
; 126623946
; 126679218
; 128294734
; 134338522
; 134972155
; 137141503
; 137259869
; 141622985
; 144205194
; 152037161
; 160964704
; 164831491
; 170465223
; 178103767
; 179225784
; 198993194
; 223439844
; 223681168
; 226486312
; 249990931
; 250170659
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0Z9NZ
- Formula
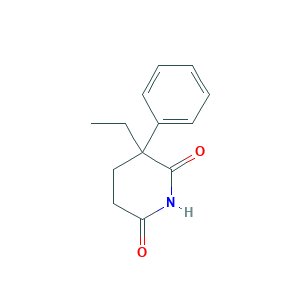
- C13H15NO2
- Canonical SMILES
- CCC1(CCC(=O)NC1=O)C2=CC=CC=C2
- InChI
- 1S/C13H15NO2/c1-2-13(10-6-4-3-5-7-10)9-8-11(15)14-12(13)16/h3-7H,2,8-9H2,1H3,(H,14,15,16)
- InChIKey
- JMBQKKAJIKAWKF-UHFFFAOYSA-N
|