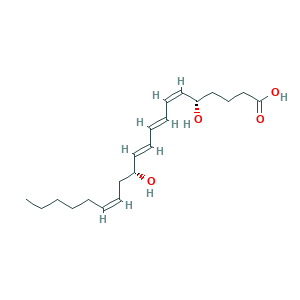
| Synonyms |
Leukotriene B4; LTB4; (5S,6Z,8E,10E,12R,14Z)-5,12-dihydroxyicosa-6,8,10,14-tetraenoic acid; LEUKOTRIENE B4; VNYSSYRCGWBHLG-AMOLWHMGSA-N; (S-(R*,S*-(E,Z,E,Z)))-5,12-Dihydroxy-6,8,10,14-eicosatetraenoic acid; 1HGW4DR56D; 5(S),12(R)-Dihydroxy-6-cis-8-trans-10-trans-14-cis-eicosatetraenoic Acid; 5,12-Dihete; 5,12-Hete; 5S,12R-dihydroxy-6Z,8E,10E,14Z-eicosatetraenoic acid; 71160-24-2; CHEBI:15647; CHEMBL65061; UNII-1HGW4DR56D; [5S,12R]-Dihydroxy-[6Z,8E,10E,14Z]-eicosatetraenoic acid
|
| Cross-matching ID |
- PubChem CID
- 5280492
- PubChem SID
-
5240
; 801682
; 4266069
; 7979751
; 8143279
; 8616293
; 14753488
; 15316444
; 24896256
; 26754791
; 26754792
; 26754793
; 26759044
; 39289598
; 47217017
; 47515561
; 47811004
; 48110702
; 48259480
; 48334747
; 49964833
; 50110780
; 53790345
; 53801088
; 57357761
; 79593894
; 92126167
; 92309780
; 99300694
; 99302212
; 103256813
; 103925923
; 104046421
; 113853357
; 126523796
; 127316819
; 127316820
; 127316821
; 127316822
; 127316823
; 127316824
; 127316825
; 127316826
; 127316827
; 127316828
; 127316829
; 127316830
; 127316831
; 134341607
; 135010128
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0O4NR
- Formula
- C20H32O4
- Canonical SMILES
- CCCCCC=CCC(C=CC=CC=CC(CCCC(=O)O)O)O
- InChI
- 1S/C20H32O4/c1-2-3-4-5-6-9-13-18(21)14-10-7-8-11-15-19(22)16-12-17-20(23)24/h6-11,14-15,18-19,21-22H,2-5,12-13,16-17H2,1H3,(H,23,24)/b8-7+,9-6-,14-10+,15-11-/t18-,19-/m1/s1
- InChIKey
- VNYSSYRCGWBHLG-AMOLWHMGSA-N
|