| General Information of Drug (ID:
DR1898) |
| Drug Name |
LY-317615
|
| Synonyms |
Enzastaurin; Enzastaurin (LY317615); LY-317615; LY317615; UC96G28EQF; 170364-57-5; 3-(1-Methyl-1H-indol-3-yl)-4-(1-(1-(pyridin-2-ylmethyl)piperidin-4-yl)-1H-indol-3-yl)-1H-pyrrole-2,5-dione; 3-(1-methyl-1H-indol-3-yl)-4-{1-[1-(pyridin-2-ylmethyl)piperidin-4-yl]-1H-indol-3-yl}-1H-pyrrole-2,5-dione; 3-(1-methylindol-3-yl)-4-[1-[1-(pyridin-2-ylmethyl)piperidin-4-yl]indol-3-yl]pyrrole-2,5-dione; AK-60903; C32H29N5O2; CHEMBL300138; DSSTox_CID_24029; DSSTox_GSID_44029; DSSTox_RID_80101; UNII-UC96G28EQF
|
| Indication |
Mantle cell lymphoma
[ICD11: 2A85]
|
Phase 3
|
[1]
|
| Structure |
|
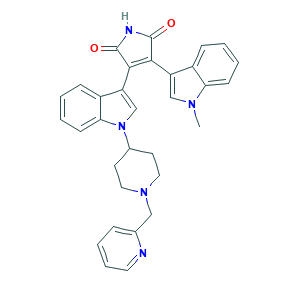 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
515.6 |
Topological Polar Surface Area |
72.2 |
| Heavy Atom Count |
39 |
Rotatable Bond Count |
5 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
4 |
| Cross-matching ID |
- PubChem CID
- 176167
- PubChem SID
-
10258360
; 14811491
; 33499842
; 50653190
; 57288469
; 92722604
; 99436960
; 103240316
; 104020050
; 104425375
; 118855345
; 124756967
; 125163772
; 125434326
; 126627253
; 126666986
; 131465124
; 134338799
; 134964387
; 135237310
; 135686208
; 135686209
; 135686224
; 135686225
; 136340265
; 136367952
; 136920322
; 137248831
; 137275839
; 142964159
; 143498272
; 144115944
; 144206918
; 152134183
; 152233034
; 152258187
; 152344347
; 152344363
; 160647023
; 160814256
; 162011915
; 162037408
; 162191234
; 162801883
; 163372664
; 163894051
; 164840960
; 170465609
; 172913635
; 174527910
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0I6VU
- Formula
- C32H29N5O2
- Canonical SMILES
- CN1C=C(C2=CC=CC=C21)C3=C(C(=O)NC3=O)C4=CN(C5=CC=CC=C54)C6CCN(CC6)CC7=CC=CC=N7
- InChI
- 1S/C32H29N5O2/c1-35-19-25(23-9-2-4-11-27(23)35)29-30(32(39)34-31(29)38)26-20-37(28-12-5-3-10-24(26)28)22-13-16-36(17-14-22)18-21-8-6-7-15-33-21/h2-12,15,19-20,22H,13-14,16-18H2,1H3,(H,34,38,39)
- InChIKey
- AXRCEOKUDYDWLF-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


