| Synonyms |
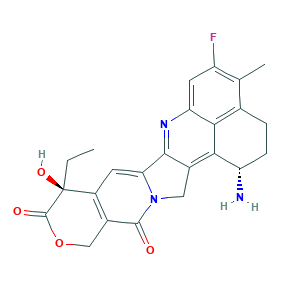
DX-8951f; Dx 8951; Exatecan; Exatecan [INN]; OC71PP0F89; (1S,9S)-1-Amino-9-ethyl-5-fluoro-1,2,3,9,12,15-hexahydro-9-hydroxy-4-methyl-10H,13H-benzo(de)pyrano(3',4':6,7)indolizino(1,2-b)quinoline-10,13-dione; 10H,13H-Benzo(de)pyrano(3',4':6,7)indolizino(1,2-b)quinoline-10,13-dione, 1-amino-9-ethyl-5-fluoro-1,2,3,9,12,15-hexahydro-9-hydroxy-4-methyl-, (1S-trans)-; 171335-80-1; C24H22FN3O4; UNII-OC71PP0F89
|
| Cross-matching ID |
- PubChem CID
- 151115
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D01FUC
- Formula
- C24H22FN3O4
- Canonical SMILES
- CCC1(C2=C(COC1=O)C(=O)N3CC4=C5C(CCC6=C5C(=CC(=C6C)F)N=C4C3=C2)N)O
- InChI
- 1S/C24H22FN3O4/c1-3-24(31)14-6-18-21-12(8-28(18)22(29)13(14)9-32-23(24)30)19-16(26)5-4-11-10(2)15(25)7-17(27-21)20(11)19/h6-7,16,31H,3-5,8-9,26H2,1-2H3/t16-,24-/m0/s1
- InChIKey
- ZVYVPGLRVWUPMP-FYSMJZIKSA-N
|