| Cross-matching ID |
- PubChem CID
- 5280363
- PubChem SID
-
3912
; 613198
; 4265953
; 7847149
; 7980284
; 8143215
; 8616237
; 14852168
; 15398784
; 26754609
; 29216066
; 39289529
; 47588758
; 47588759
; 48258963
; 48415906
; 50026801
; 50110092
; 53789218
; 56311306
; 56313004
; 56313208
; 57357710
; 77668180
; 92308787
; 92309910
; 99300824
; 99302342
; 103214323
; 103943633
; 104046437
; 113853160
; 124892254
; 124892255
; 125307573
; 126662422
; 134340899
; 134978425
; 135651540
; 137006907
; 142063920
; 144205330
; 162205063
; 175268163
; 175607780
; 176484109
; 179116866
; 211534905
; 223673680
; 226412676
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D00HTY
- Formula
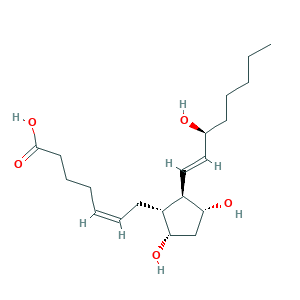
- C20H34O5
- Canonical SMILES
- CCCCCC(C=CC1C(CC(C1CC=CCCCC(=O)O)O)O)O
- InChI
- 1S/C20H34O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-19,21-23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,18-,19+/m0/s1
- InChIKey
- PXGPLTODNUVGFL-YNNPMVKQSA-N
|