Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR2000) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Acenaphthenol
|
|||||
| Synonyms |
GEO-00001; MXUCIEHYJYRTLT-UHFFFAOYSA-; MXUCIEHYJYRTLT-UHFFFAOYSA-N; SCHEMBL345448; W-200056; 1,2-Dihydro-1-acenaphthylenol; 1,2-dihydroacenaphthylen-1-ol; 1-ACENAPHTHENOL; 1-Acenaphthalenol; 1-Acenaphthenol, 99%; 1-Acenaphthylenol, 1,2-dihydro-; 1-Acenaphthylenol,1,2-dihydro-; 1-Acenaphthylenol,2-dihydro-; 1-Hydroxyacenaphthene; 28807-94-5; 7-Acenaphthenol; AC1L2L0C; AC1Q7A0H; ACMC-1B4IN; Acenaphthen-1-ol; Acenaphthene-1-ol; 1-Acenaphthenol; Acenaphthenol-1; Acenaphthylenol, 1,2-dihydro-; DTXSID20951449; HMS1666F13
|
|||||
| Indication | Discovery agent | Investigative | [1] | |||
| Structure |
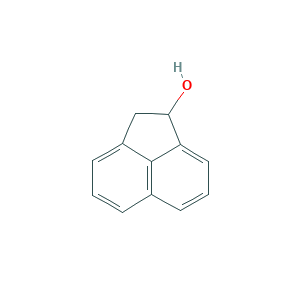 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 170.21 | Topological Polar Surface Area | 20.2 | ||
| Heavy Atom Count | 13 | Rotatable Bond Count | 0 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 1 | |||
| Cross-matching ID | ||||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||
| Experimental Enzyme Kinetic Data of This Drug | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

