| Synonyms |
E-Retinal; RR725D715M; Retinal, all-trans-; Retinaldehyde (VAN); Retinene 1; Retinyl aldehyde; TOCOPHEROL, ALPHA; Vitamin A1 aldehyde; all-E-Retinal; all-trans-Retinal; all-trans-Retinaldehyde; all-trans-Retinene; all-trans-Vitamin A aldehyde; axerophthal; retinal; retinaldehyde; retinene; trans-Retinal; trans-Vitamin A aldehyde; vitamin A aldehyde; .alpha.-Retinene; 116-31-4; BRN 1914183; CHEBI:17898; EINECS 204-135-8; MFCD00001550; NCYCYZXNIZJOKI-OVSJKPMPSA-N; NSC 122757; NSC 626581; NSC626581; UNII-RR725D715M
|
| Cross-matching ID |
- PubChem CID
- 638015
- PubChem SID
-
3136
; 3666
; 83356
; 228445
; 416401
; 416402
; 493006
; 589152
; 3157260
; 8143959
; 8704598
; 10504630
; 10524847
; 12276012
; 14775466
; 17388687
; 24702066
; 24899355
; 24899382
; 43760279
; 48424039
; 48425697
; 49854777
; 53790090
; 53812631
; 57407725
; 77047160
; 91615887
; 103282386
; 103847254
; 113528124
; 113584862
; 117624017
; 117682346
; 124890043
; 124890044
; 124890045
; 124890046
; 124890047
; 126523991
; 126637303
; 134975373
; 135651434
; 137004766
; 137005733
; 142478968
; 144208203
; 162219414
; 164806111
; 175265990
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D05SOU
- Formula
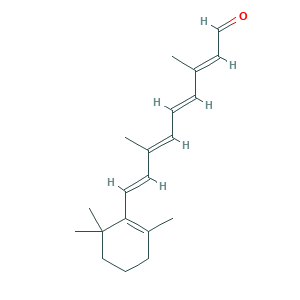
- C20H28O
- Canonical SMILES
- CC1=C(C(CCC1)(C)C)C=CC(=CC=CC(=CC=O)C)C
- InChI
- 1S/C20H28O/c1-16(8-6-9-17(2)13-15-21)11-12-19-18(3)10-7-14-20(19,4)5/h6,8-9,11-13,15H,7,10,14H2,1-5H3/b9-6+,12-11+,16-8+,17-13+
- InChIKey
- NCYCYZXNIZJOKI-OVSJKPMPSA-N
|