| Synonyms |
Cytidin; Cytosine riboside; Cytosine, 1-beta-D-ribofuranosyl-; Zytidin; beta-D-Ribofuranoside, cytosine-1; cytidine; 1-beta-D-Ribofuranosylcytosine; 1-beta-Ribofuranosylcytosine; 1beta-2'-Ribofuranosylcytosine, d-; 1beta-Ribofuranosylcytosine; 2(1H)-Pyrimidinone, 4-amino-1-beta-D-ribofuranosyl-; 4-Amino-1-beta-D-ribofuranosyl-2(1H)-pyrimidinone; 4-Amino-1beta-D-ribofuranosyl-2(1H)-pyrimidinone; 65-46-3; CHEBI:17562; Cyd; EINECS 200-610-9; MFCD00006545; NSC 20258; UNII-5CSZ8459RP
|
| Cross-matching ID |
- PubChem CID
- 6175
- PubChem SID
-
2561
; 3758
; 610624
; 833276
; 841097
; 3132638
; 8144101
; 8153876
; 11110524
; 12109173
; 15342347
; 24892387
; 24892710
; 25621293
; 26754267
; 29225173
; 49684259
; 49693276
; 49834502
; 50036797
; 51092061
; 56459360
; 76890969
; 81044585
; 85098495
; 85165010
; 85230757
; 87565639
; 88211484
; 92297505
; 93576752
; 99246698
; 99381411
; 99453898
; 103313321
; 103823300
; 104223697
; 104311310
; 117588066
; 124558522
; 125264648
; 126523004
; 126635400
; 127303743
; 127303744
; 127303745
; 127303746
; 127303747
; 127303748
; 127303749
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0E7ES
- Formula
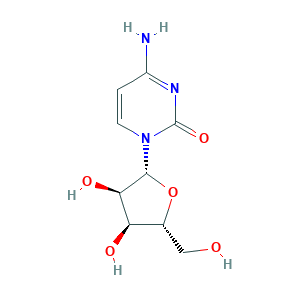
- C9H13N3O5
- Canonical SMILES
- C1=CN(C(=O)N=C1N)C2C(C(C(O2)CO)O)O
- InChI
- 1S/C9H13N3O5/c10-5-1-2-12(9(16)11-5)8-7(15)6(14)4(3-13)17-8/h1-2,4,6-8,13-15H,3H2,(H2,10,11,16)/t4-,6-,7-,8-/m1/s1
- InChIKey
- UHDGCWIWMRVCDJ-XVFCMESISA-N
|