Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR2120) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Phenylglyoxal
|
|||||
| Synonyms |
Benzeneacetaldehyde, .alpha.-oxo-; OJUGVDODNPJEEC-UHFFFAOYSA-N; Oxo(phenyl)acetaldehyde; Oxo-phenyl-acetaldehyde; Phenyl glyoxal; alpha-oxobenzeneacetaldehyde; benzoylcarboxaldehyde; benzoylformaldehyde; glyoxal, phenyl-; phenylethanedione; phenylglyoxal; 1074-12-0; 2-oxo-2-phenyl-acetaldehyde; 2-oxo-2-phenylacetaldehyde; AI3-22132; BRN 1854721; CCRIS 966; CHEBI:88868; EINECS 214-036-1; MFCD00006959; N45G3015PA; NSC 156299; NSC 26909; NSC627436; UNII-N45G3015PA; benzeneacetaldehyde, alpha-oxo-, monohydrate
|
|||||
| Indication | Discovery agent | Investigative | [1] | |||
| Structure |
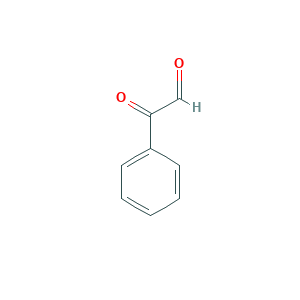 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 134.13 | Topological Polar Surface Area | 34.1 | ||
| Heavy Atom Count | 10 | Rotatable Bond Count | 2 | |||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 2 | |||
| Cross-matching ID | ||||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||
| Experimental Enzyme Kinetic Data of This Drug | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

