| Cross-matching ID |
- PubChem CID
- 17473
- PubChem SID
-
3467
; 837291
; 841355
; 6436278
; 7890983
; 8145724
; 8163087
; 15932622
; 24902502
; 29285212
; 53776881
; 53788522
; 57304739
; 57329784
; 78260467
; 85165096
; 103516229
; 104342557
; 125003537
; 125003542
; 125003544
; 125267527
; 126523689
; 134981783
; 135651587
; 137244581
; 160660760
; 163727003
; 198976871
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0O8AN
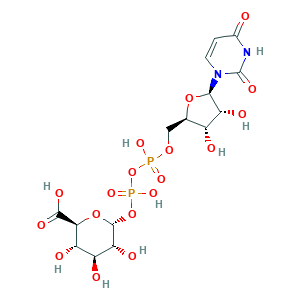
- Formula
- C15H22N2O18P2
- Canonical SMILES
- C1=CN(C(=O)NC1=O)C2C(C(C(O2)COP(=O)(O)OP(=O)(O)OC3C(C(C(C(O3)C(=O)O)O)O)O)O)O
- InChI
- 1S/C15H22N2O18P2/c18-5-1-2-17(15(26)16-5)12-9(22)6(19)4(32-12)3-31-36(27,28)35-37(29,30)34-14-10(23)7(20)8(21)11(33-14)13(24)25/h1-2,4,6-12,14,19-23H,3H2,(H,24,25)(H,27,28)(H,29,30)(H,16,18,26)/t4-,6-,7+,8+,9-,10-,11+,12-,14-/m1/s1
- InChIKey
- HDYANYHVCAPMJV-LXQIFKJMSA-N
|