| General Information of Drug (ID:
DR2284) |
| Drug Name |
Ethoxzolamide
|
| Synonyms |
Ethamide; Ethoxazolamide; Ethoxyzolamide; Ethoxzolamide (EZA); Etoxzolamide; Glaucotensil; Mingoral; Redupresin; U-4191; ethoxzolamide; Cardrase; Cardrase (TN); Diuretic C; 2-Benzothiazolesulfonamide, 6-ethoxy-; 452-35-7; 6-(ethyloxy)-1,3-benzothiazole-2-sulfonamide; 6-Ethoxy-1,3-benzothiazole-2-sulfonamide; 6-Ethoxy-2-benzothiazolesulfonamide; 6-Ethoxy-benzothiazole-2-sulfonic acid amide; 6-Ethoxybenzo[d]thiazole-2-sulfonamide; 6-Ethoxybenzothiazole-2-sulfonamide; 6-Ethoxybenzothiazole-2-sulphonamide; 6-Ethoxyzolamide; 6-ethoxy-1,3-benzothiazole-2-sulfonamide; AI3-50805; BRN 0212240; C9H10N2O3S2; CHEMBL18; EINECS 207-199-5; EZL; HSDB 3268; MLS000028637; NSC 10679; UNII-Z52H4811WX
|
| Indication |
Glaucoma
[ICD11: 9C61]
|
Approved
|
[1]
|
| Structure |
|
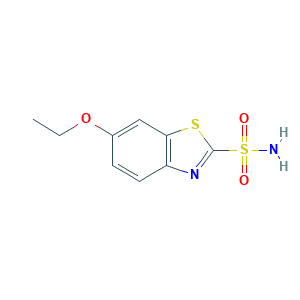 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
258.3 |
Topological Polar Surface Area |
119 |
| Heavy Atom Count |
16 |
Rotatable Bond Count |
3 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
6 |
| Cross-matching ID |
- PubChem CID
- 3295
- PubChem SID
-
75836
; 840267
; 855751
; 893338
; 5242023
; 7849499
; 8152095
; 10523205
; 15213608
; 24860121
; 29215007
; 29222433
; 46509023
; 46511452
; 48415980
; 49681475
; 49890761
; 50100966
; 56422188
; 57280084
; 57280086
; 57288316
; 57304388
; 57321712
; 80657483
; 85176911
; 85306595
; 87557627
; 92308013
; 92308213
; 92729651
; 95614375
; 96021935
; 99444198
; 103048393
; 103164710
; 104171380
; 104303092
; 124587685
; 124800633
; 124883557
; 124883558
; 124897844
; 125637519
; 126671832
; 128865742
; 134337889
; 134975323
; 135378086
; 137004941
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D07INV
- Formula
- C9H10N2O3S2
- Canonical SMILES
- CCOC1=CC2=C(C=C1)N=C(S2)S(=O)(=O)N
- InChI
- 1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13)
- InChIKey
- OUZWUKMCLIBBOG-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


