| General Information of Drug (ID:
DR2413) |
| Drug Name |
Cefadroxil
|
| Synonyms |
Cefadroxil (JP15); Cefadroxil anhydrous; Cefadroxilo; Cefadroxilo [INN-Spanish]; Cefadroxilum; Cefadroxilum [INN-Latin]; Cephadroxil; Curisafe (TN); D-Cefadroxil; BL-S 578; BL-S578; MJF-11567-3; Sumacef; Sumacef (TN); (6R,7R)-7-((R)-2-Amino-2-(p-hydroxyphenyl)acetamido)-3-methyl-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid; (6R,7R)-7-[[(2R)-2-amino-2-(4-hydroxyphenyl)acetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-{[(2R)-2-amino-2-(4-hydroxyphenyl)acetyl]amino}-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; 7beta-{[(2R)-2-amino-2-(4-hydroxyphenyl)acetyl]amino}-3,4-didehydrocepham-4-carboxylic acid; CDX; S 578; S-578
|
| Indication |
Acute tonsillitis
[ICD11: CA03]
|
Approved
|
[1]
|
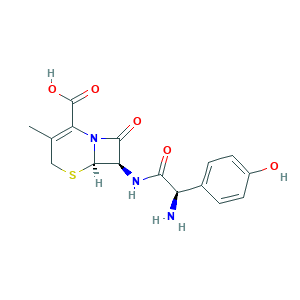
| Structure |
|
 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
363.4 |
Topological Polar Surface Area |
158 |
| Heavy Atom Count |
25 |
Rotatable Bond Count |
4 |
| Hydrogen Bond Donor Count |
4 |
Hydrogen Bond Acceptor Count |
7 |
| Cross-matching ID |
- PubChem CID
- 47965
- PubChem SID
-
9095
; 7847323
; 7978892
; 8149235
; 10990583
; 11335584
; 11360823
; 11362958
; 11365520
; 11368082
; 11373871
; 11376244
; 11461795
; 11466462
; 11467582
; 11483750
; 11486123
; 11487903
; 11492081
; 11493918
; 14852631
; 16050996
; 24892921
; 25622160
; 34712894
; 46509128
; 47440185
; 47515253
; 47515254
; 47959665
; 48035040
; 48334422
; 48415709
; 49699087
; 50124273
; 57654040
; 75439952
; 85279381
; 87322627
; 90451718
; 92125414
; 93576133
; 93576712
; 99301497
; 103620148
; 103914339
; 104133801
; 104354273
; 121363085
; 124766005
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0X9ZC
- Formula
- C16H17N3O5S
- Canonical SMILES
- CC1=C(N2C(C(C2=O)NC(=O)C(C3=CC=C(C=C3)O)N)SC1)C(=O)O
- InChI
- 1S/C16H17N3O5S/c1-7-6-25-15-11(14(22)19(15)12(7)16(23)24)18-13(21)10(17)8-2-4-9(20)5-3-8/h2-5,10-11,15,20H,6,17H2,1H3,(H,18,21)(H,23,24)/t10-,11-,15-/m1/s1
- InChIKey
- BOEGTKLJZSQCCD-UEKVPHQBSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


