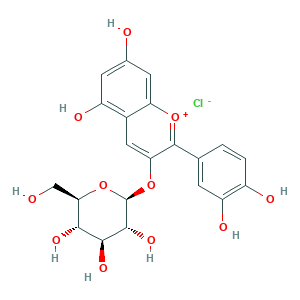
| Synonyms |
Asterin; CYANIDIN 3-GLUCOSIDE CHLORIDE; Chrysanthemin (6CI,8CI); Chrysontemin; Cyanidin 3-O-beta-D-glucoside; Cyanidin 3-glucoside; Cyanidin 3-monoglucoside; Cyanidin-3-glucoside chloride; Cyanidine 3-glucoside; Cyanidol 3-glucoside; Glucocyanidin; Kuromanin (chloride); Kuromanin chloride; Kuromanine; cyanidin-3-O-glucoside; cyanidin-3-glucoside; cyanidin-3-o-glucoside chloride; 2-(3,4-Dihydroxyphenyl)-3-(beta-D-glucopyranosyloxy)-5,7-dihydroxy-1-benzopyrylium chloride; 7084-24-4; 8X15R84UEM; EINECS 230-384-7; UNII-8X15R84UEM
|
| Cross-matching ID |
- PubChem CID
- 197081
- CAS Number
-
- Formula
- C21H21ClO11
- Canonical SMILES
- C1=CC(=C(C=C1C2=[O+]C3=CC(=CC(=C3C=C2OC4C(C(C(C(O4)CO)O)O)O)O)O)O)O.[Cl-]
- InChI
- 1S/C21H20O11.ClH/c22-7-16-17(27)18(28)19(29)21(32-16)31-15-6-10-12(25)4-9(23)5-14(10)30-20(15)8-1-2-11(24)13(26)3-8;/h1-6,16-19,21-22,27-29H,7H2,(H3-,23,24,25,26);1H/t16-,17-,18+,19-,21-;/m1./s1
- InChIKey
- YTMNONATNXDQJF-UBNZBFALSA-N
|