Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR2498) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
HY-N2258
|
|||||
| Synonyms |
Citrifolioside; Poncirin; Poncirin, analytical standard; SCHEMBL123069; SureCN123069; ZINC8234320; (2S)-poncirin; 14941-08-3; 8MUY4P95B4; AKOS037514792; CHEBI:66773; CHEMBL451050; CP0093; DTXSID00933642; EINECS 239-020-1; HY-N2258; Isosakuranetin 7-O-neohesperidoside; NLAWPKPYBMEWIR-SKYQDXIQSA-N; Isosakuranetin-7-O-beta-D-neohesperidoside; Isosakuranetin-7-O-neohesperidoside; NCGC00163611-01; UNII-8MUY4P95B4; s9165
|
|||||
| Indication | Anaesthesia [ICD11: 8E22] | Preclinical | [1] | |||
| Structure |
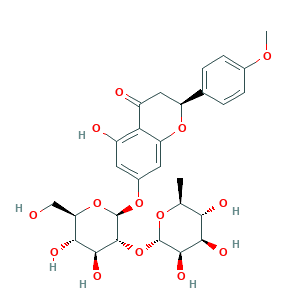 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 594.6 | Topological Polar Surface Area | 214 | ||
| Heavy Atom Count | 42 | Rotatable Bond Count | 7 | |||
| Hydrogen Bond Donor Count | 7 | Hydrogen Bond Acceptor Count | 14 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
| Experimental Enzyme Kinetic Data of This Drug | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

