| Synonyms |
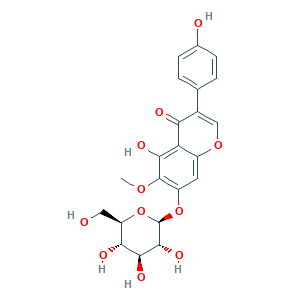
Shekanin; Tectoridin; Tectoridin (7CI,8CI); ZINC4098747; 4',5-Dihydro-6-methoxy-7-(o-glucoside)isoflavone; 4-18-00-03312 (Beilstein Handbook Reference); 4H-1-Benzopyran-4-one, 7-(beta-D-glucopyranosyloxy)-5-hydroxy-3-(4-hydroxyphenyl)-6-methoxy-; 5-hydroxy-3-(4-hydroxyphenyl)-6-methoxy-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one; 611-40-5; 968X515NZH; BRN 0068384; CHEBI:9428; CHEMBL520214; CTK8F0927; DTXSID70209982; CNOURESJATUGPN-UDEBZQQRSA-N; SCHEMBL241734; HY-N0791; UNII-968X515NZH
|
| Cross-matching ID |
- PubChem CID
- 5281810
- ChEBI ID
-
- CAS Number
-
- Formula
- C22H22O11
- Canonical SMILES
- COC1=C(C=C2C(=C1O)C(=O)C(=CO2)C3=CC=C(C=C3)O)OC4C(C(C(C(O4)CO)O)O)O
- InChI
- 1S/C22H22O11/c1-30-21-13(32-22-20(29)19(28)17(26)14(7-23)33-22)6-12-15(18(21)27)16(25)11(8-31-12)9-2-4-10(24)5-3-9/h2-6,8,14,17,19-20,22-24,26-29H,7H2,1H3/t14-,17-,19+,20-,22-/m1/s1
- InChIKey
- CNOURESJATUGPN-UDEBZQQRSA-N
|