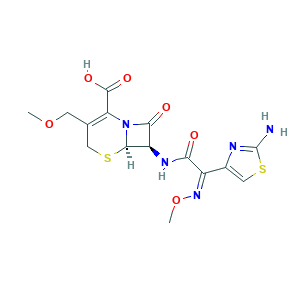
| Synonyms |
Cefpodoxim acid; Cefpodoxima; Cefpodoxima [Spanish]; Cefpodoxime (INN); Cefpodoxime [INN:BAN]; Cefpodoximum; Cefpodoximum [Latin]; Epoxim; Epoxim (TN); RU 51807; Vantin (TN); (6R,7R)-7-({(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-[(methyloxy)imino]acetyl}amino)-3-[(methyloxy)methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-[[(2E)-2-(2-amino-1,3-thiazol-4-yl)-2-methoxyiminoacetyl]amino]-3-(methoxymethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-[[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-methoxyiminoacetyl]amino]-3-(methoxymethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; 7-{[(2-amino-1,3-thiazol-4-yl)(methoxyimino)acetyl]amino}-3-(methoxymethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; CPDX
|
| Cross-matching ID |
- PubChem CID
- 6335986
- PubChem SID
-
10314
; 7978883
; 14832042
; 14880676
; 23947634
; 42980683
; 46504897
; 48415725
; 49835234
; 51091954
; 79167407
; 87305645
; 96099266
; 103639168
; 104133802
; 114466102
; 124766016
; 134337752
; 135013061
; 137002897
; 138999736
; 140396441
; 160964685
; 163409420
; 163847698
; 175268415
; 179151199
; 196378890
; 226414761
; 241157558
; 252347160
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0W6CA
- Formula
- C15H17N5O6S2
- Canonical SMILES
- COCC1=C(N2C(C(C2=O)NC(=O)C(=NOC)C3=CSC(=N3)N)SC1)C(=O)O
- InChI
- 1S/C15H17N5O6S2/c1-25-3-6-4-27-13-9(12(22)20(13)10(6)14(23)24)18-11(21)8(19-26-2)7-5-28-15(16)17-7/h5,9,13H,3-4H2,1-2H3,(H2,16,17)(H,18,21)(H,23,24)/b19-8-/t9-,13-/m1/s1
- InChIKey
- WYUSVOMTXWRGEK-HBWVYFAYSA-N
|