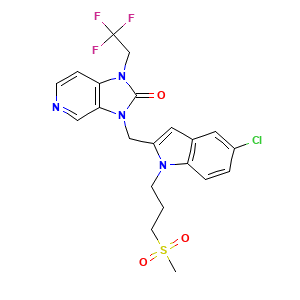
| Synonyms |
Rilematovir; JNJ-678; UNII-NQ99E8OH3P; NQ99E8OH3P; 1383450-81-4; 3-((5-chloro-1-(3-(methylsulfonyl)propyl)-1H-indol-2-yl)methyl)-1-(2,2,2-trifluoroethyl)-1,3-dihydro-2H-imidazo[4,5-c]pyridin-2-one; 3-[[5-Chloranyl-1-(3-Methylsulfonylpropyl)indol-2-Yl]methyl]-1-[2,2,2-Tris(Fluoranyl)ethyl]imidazo[4,5-C]pyridin-2-One; Rilematovir [INN]; CHEMBL4437054; SCHEMBL17529353; HY-112180; CS-0043622; C(CCN1C(=CC2=CC(=CC=C12)Cl)CN1C2=C(N(C1=O)CC(F)(F)F)C=CN=C2)S(=O)(=O)C; 2H-Imidazo(4,5-C)pyridin-2-one, 3-((5-chloro-1-(3-(methylsulfonyl)propyl)-1H-indol-2-yl)methyl)-1,3-dihydro-1-(2,2,2-trifluoroethyl)-; 3-((5-Chloro-1-(3-methylsulfonylpropyl)indol-2-yl)methyl)-1-(2,2,2-trifluoroethyl)imidazo(4,5-C)pyridin-2-one; 6YA
|
| Cross-matching ID |
- PubChem CID
- 118892432
- CAS Number
-
- TTD Drug ID
- DRH80V
- Formula
- C21H20ClF3N4O3S
- Canonical SMILES
- CS(=O)(=O)CCCN1C2=C(C=C(C=C2)Cl)C=C1CN3C4=C(C=CN=C4)N(C3=O)CC(F)(F)F
- InChI
- InChI=1S/C21H20ClF3N4O3S/c1-33(31,32)8-2-7-27-16(10-14-9-15(22)3-4-17(14)27)12-28-19-11-26-6-5-18(19)29(20(28)30)13-21(23,24)25/h3-6,9-11H,2,7-8,12-13H2,1H3
- InChIKey
- GTQTUABHRCWVLL-UHFFFAOYSA-N
|